Abstract
Focal adhesion kinase (FAK) is a protein tyrosine kinase that regulates cellular adhesion, motility, proliferation and survival in various types of cells. Interestingly, FAK is activated and/or overexpressed in advanced cancers, and promotes cancer progression and metastasis. For this reason, FAK became a potential therapeutic target in cancer, and small molecule FAK inhibitors have been developed and are being tested in clinical phase trials. These inhibitors have demonstrated to be effective by inducing tumor cell apoptosis in addition to reducing metastasis and angiogenesis. Furthermore, several genetic FAK mouse models have made advancements in understanding the specific role of FAK both in tumors and in the tumor environment. In this review, we discuss FAK inhibitors as well as genetic mouse models to provide mechanistic insights into FAK signaling and its potential in cancer therapy.
Keywords: FAK, cancer, FAK inhibitors, genetic mouse model, metastasis
Introduction
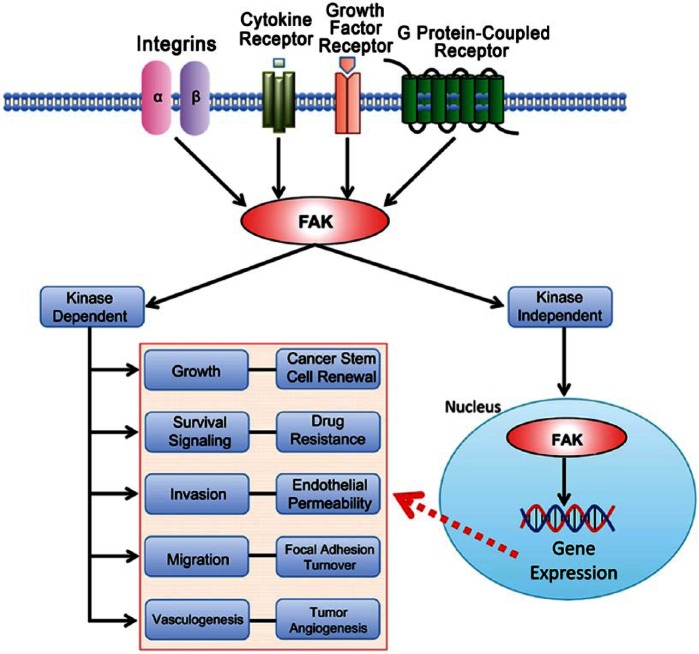
Focal adhesion kinase (FAK) serves as a fundamental intracellular mediator of extracellular changes, such as extracellular matrix (ECM) remodeling, nutrient availability, and growth factors (Fletcher and Mullins 2010). FAK is a non-receptor protein tyrosine kinase that is activated by interactions with integrins, growth factor receptors, G protein-coupled receptors, and cytokine receptors (Fig. 1) (Lim et al. 2010; Mitra et al. 2005), and it is known to have a pivotal role in the regulation of cell adhesion, motility, proliferation, and survival in many cell types (Peng and Guan 2011).
Figure 1.
FAK signaling pathways in cancer. Integrins, cytokine receptors (CR), growth factor receptors (GFR), and G protein-coupled receptors (GPCR) are cell-surface transmembrane receptors that relay extracellular signals to FAK. FAK activation triggers subsequent signaling cascades in various cell processes: survival signaling, growth, angiogenesis, migration, and invasion. In cancers, FAK is overexpressed and activated and it is likely that the FAK signaling pathway is deregulated in cancer cells. FAK functions in both kinase-dependent and -independent manners. Kinase-inhibited FAK goes to the nucleus and potentially regulates gene expression to affect cancer progression.
The FAK gene is mapped on human chromosome 8q24.3 and ubiquitously expressed in most cells (Schaller et al. 1994; Zachary 1997). FAK itself does not act as an oncogene, but the FAK protein is overexpressed in many cancers (Table 1). FAK gene (gene symbol PTK2) amplification in the chromosome (Zhao and Guan 2009) or its upregulation by tumor-related transcription factors can contribute to FAK overexpression and activation in cancer (Cance and Golubovskaya 2008; Corsi et al. 2006). Activated FAK plays an important role as a key signal mediator in tumor progression and metastasis, suggesting that FAK is a potential target for cancer therapeutics (Fig. 1).
Table 1.
FAK Overexpression in Cancer.
| Tumor Type | Occurrences* | References |
|---|---|---|
| Head and neck squamous cell carcinoma | ~62% | (Canel et al. 2006; He et al. 2006) |
| Neuroblastoma | ~73% | (Beierle et al. 2008) |
| Breast cancer | ~25-77% | (Cance et al. 2000b; Lark et al. 2005) |
| Colorectal cancer | ~40-86% | (Cance et al. 2000b; Theocharis et al. 2003) |
| Pancreatic cancer | ~48% | (Furuyama et al. 2006) |
| Esophageal cancer | ~59% | (Miyazaki et al. 2003) |
| Lung cancer | ~44% | (Wang et al. 2005) |
| Small-cell lung cancer | ~59% | (Ocak et al. 2012) |
| Ovarian cancer | ~68% | (Sood et al. 2004) |
Occurrences were based on immunohistochemistry analyses.
In a recent study, FAK-Del33 (deletion of exon 33) was identified, and its mutation increased cell migration by promoting tyrosine 397 (Y397) autophosphorylation in human breast cancer cells (Fang et al. 2014); this suggested that genetic FAK mutations can play an important role in tumor progression and motility. Alternative splicing generates various FAK isoforms in cancers (Fang et al. 2014), which makes the study of FAK more complicated.
As higher FAK activity contributes to tumor proliferation and metastasis, efforts have been made by pharmaceutical companies to develop FAK inhibitors. Several small molecule FAK inhibitors have been tested in both preclinical and clinical phase trials.
In this review, we discuss therapeutic FAK inhibitors for cancer therapy and their recent status. Small molecule FAK inhibitors have been reported to affect both tumor cells and stroma. Therefore, this review also covers recent advances in FAK research with genetic animal models to elucidate unidentified functions of FAK along the various stages of cancer progression and in malignant phenotypes.
FAK Structure and Function
FAK functions can be separated into two main categories: kinase-dependent and kinase-independent. FAK kinase-dependent functions are often associated with integrin-related signaling at focal adhesions where FAK plays an important role in cellular migration and adhesion in both normal and cancer cells (Fig.1) (Cary et al. 1996; Parsons 2003). FAK also functions as a scaffold and participates in protein–protein interactions through its kinase-independent functions. Interestingly, FAK has been shown to localize to the nucleus and interact directly with p53 to promote cell proliferation and survival through p53 degradation (Golubovskaya et al. 2005; Lim et al. 2008b); this demonstrates a new function of FAK in the nucleus that occurs in a kinase-independent manner.
N-terminal Domain
FAK can be divided into three domains: N-terminal FERM (band 4.1, ezrin, radixin, moesin), central kinase, and C-terminal domains (Fig. 2). The unique FAK FERM region is non-catalytic and a similar FERM motif can be found in the Janus kinase (JAK), another tyrosine kinase (Ihle 1995). The FAK FERM domain structure was recently solved and consists of three distinct subdomains (F1, F2, and F3) (Ceccarelli et al. 2006). Growth factor receptors and chemokine receptors are membrane proteins that bind to the FERM domain (Chen et al. 2011; Jung and McCarty 2012; Sieg et al. 2000). The FAK FERM domain contains a nuclear localization sequence (NLS) (Lim et al. 2008b) and, recently, several nuclear proteins have been identified as FERM-binding proteins. The F1 subdomain of FERM interacts with p53, which promotes cell survival (Lim et al. 2008a). FAK kinase-independent activity is associated with FERM-mediated survival and is based on the presence of the NLS at the F2 subdomain. Lim et al. (2008b) also showed that the FERM F3 subdomain interacts with Mdm-2, which enhances p53 ubiquitination. The FERM domain also directly binds to GATA4, a transcription factor, upon FAK inhibition and it enhances GATA4 degradation, resulting in the regulation of anti-inflammation signaling through vascular cell adhesion molecule-1 (VCAM-1) expression (Lim et al. 2012). These studies showed that the FAK FERM domain has an important role in cellular regulation by binding to membrane- and adhesion-associated proteins and transcription factors (Lim et al. 2012; Lim 2013). A basic amino acid cluster in the F2 subdomain contains a kinase domain-binding sequence (KDBS), which is critical in regulating the kinase activity via an auto-inhibitory mechanism (Fig. 2) (Frame et al. 2010; Lietha et al. 2007).
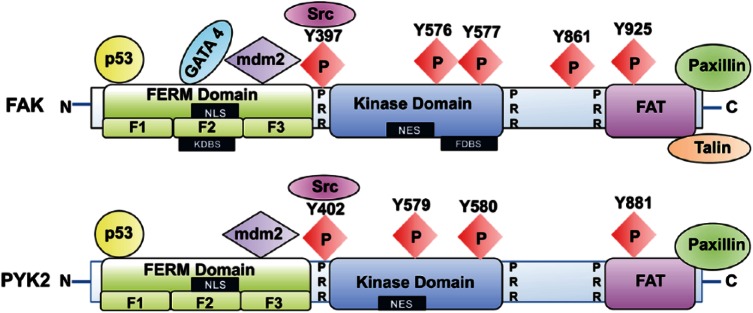
Figure 2.
Schematic structure of FAK and Pyk2. FAK and Pyk2 contain N- and C-terminal domains and a central kinase domain. The N-terminal domain of FAK and Pyk2 contains the FERM (band 4.1, ezrin, radixin, moesin) domain, which is composed of subdomains F1, F2, and F3. FAK is autophosphorylated at tyrosine Y397, situated at the end of the N-terminus and the beginning of the kinase domain, and there are four additional tyrosine phosphorylation sites on the protein (Y576, Y577, Y861, and Y925). Upon Y397 phosphorylation, Src tyrosine kinase binds to the Y397 site, and the FAK-Src complex further phosphorylates the activation loop Y576/577 sites, which are important for the catalytic activity of the kinase. Many regulatory proteins, such as p53, Mdm-2 and growth factor receptors, bind to the FAK FERM domain. The C-terminal domain of FAK contains a FAT (focal adhesion targeting) domain, which includes two tyrosine sites, Y861 and Y925; phosphorylation of FAK at these sites creates binding sites for SH2 (Src homology-2) domains, whereas proline-rich regions (PRR) provide binding sites for SH3 domain-containing proteins. The FAT domain associates with integrin receptors indirectly via focal adhesion proteins, such as paxillin and talin. Pyk2 contains four major tyrosine phosphorylation sites (Y402, Y597, Y580, and Y881). The Pyk2 autophosphorylation site at Y402 or Y881 facilitates binding of SH2 domain-containing protein. Unlike FAK, the FAT domain of Pyk2 binds to paxillin but not talin. Abbreviations: NLS, nuclear localization sequence; NES, nuclear export sequence; KDBS, kinase domain binding site; FDBS, FERM domain binding site.
Kinase Domain
The FAK kinase domain resides in the middle of FAK (Parsons 2003) and contains the activation loop, tyrosine (Y) sites Y576 and Y577, which can be phosphorylated by Src; these sites ultimately regulate FAK kinase activity (Chan et al. 1999). Integrin, growth factor, and chemokine receptor stimulation result in the rapid autophosphorylation at Y397 (Calalb et al. 1995). The autophosphorylation site at Y397 and two other tyrosine phosphorylation sites at the activation loop, Y576 and Y577, are highly conserved within the catalytic kinase domain of protein tyrosine kinases (Owen et al. 1999). Phosphorylation-specific antibodies against phospho-Y397 and -Y576 (Life Technologies, #44-625G and 700013) are highly specific and present no cross-reactivity with Pyk2; these are therefore commonly used to monitor FAK activation during immunoblotting and immunohistochemistry.
Structural analysis of FAK FERM with the kinase domain revealed that the FAK FERM domain interacts and blocks the kinase domain, resulting in an auto-inhibited conformation (Lietha et al. 2007). The FERM domain binds directly to the kinase domain, centered at phenylalanine (F) 596 (Frame et al. 2010) (Fig. 2) and thus effectively blocks any catalytic activity. The structure further indicates that the intra-molecular interaction between FAK FERM and the kinase domain results in the “closed confirmation” of FAK, and the interaction of FERM with other activators can induce conformational changes that release the kinase domain from this closed conformation and subsequently leads to FAK activation (Cooper et al. 2003). Indeed, phosphatidyl inositol 4,5 bisphosphate (PIP2) binding to the FERM F2 basic cluster region opens the closed kinase domain and results in an active conformation of the protein (Barsukov et al. 2003).
C-terminal Domain
The C-terminal domain (CD) is characterized by two proline-rich sequences and the FAT (focal adhesion targeting) sequence (Fig. 2) (Parsons 2003). The CD of FAK, similar to the FERM domain, participates in various protein–protein interactions. As FAK is not directly linked to integrin cytoplasmic tails, the FAT sequence directs FAK to focal adhesions via paxillin and talin. FAK dimerization via the interaction between FAT and basic residues in the FERM F2 subdomain has been reported (Brami-Cherrier et al. 2014). This FAT–FERM interaction plays an important role in FAK activation and nuclear localization through modulation of paxillin binding (Brami-Cherrier et al. 2014). The exogenous expression of the FAK CD causes cell rounding, a loss of adhesion, and apoptosis in human cancer cells (Xu et al. 1998), suggesting that the FAK CD may inhibit endogenous FAK function. There are several additional trans-phosphorylation sites, Y861 and Y925, in the FAK CD, which provides binding sites for SH2 (Src homology-2) domain-containing proteins (Calalb et al. 1995; Schlaepfer et al. 1998).
FAK Functions in Cancer
FAK Gene Amplification
FAK protein is overexpressed in various cancers, including ovarian, cervical, kidney, lung, pancreatic, brain, colon, breast, and skin cancer (Golubovskaya et al. 2009) (Table 1). In addition, studies have confirmed a correlation between FAK overexpression levels and poorer clinical prognosis in several types of human tumors (Yom et al. 2011). One important mechanism of FAK overexpression is gene amplification, and increased FAK mRNA—which accounts for increased FAK expression—is also found in invasive and metastatic tumors, including head and neck, lung, colon, and breast cancer (Agochiya et al. 1999; Sulzmaier et al. 2014; Weiner et al. 1993).
Recently, several studies specifically examined FAK gene amplification in human tumors. FAK gene amplification was found to be a poor prognostic factor as well as an important indicator in gastric cancer (Park et al. 2010) and in invasive breast cancer (Yom et al. 2011). In contrast, correlations between FAK gene amplification and tumor progression in certain other human tumors have been inconsistent. Whereas overexpression of FAK in head and neck squamous cell carcinoma is independent of FAK gene amplification (Canel et al. 2006), there are still significant correlations between some invasive cancers and high FAK mRNA levels.
Cancer Initiation and Cell Survival
FAK expression has been linked to the initiation and survival of cancer cells and was first shown to prevent cell apoptosis in Madin-Darby canine kidney (MDCK) cells (Frisch et al. 1996). Genetic FAK deletion in skin keratinocytes has been shown to suppress dimethyl benzanthracene (DMBA)-induced skin cancer formation. In addition, deletion of FAK in keratinocytes induces cell apoptosis in vitro and in vivo (McLean et al. 2004a), and mammary tissue-specific FAK knockout with p53 deletion has been shown to reduce mammary tumor formation (van Miltenburg et al. 2014). FAK inhibition by FAK siRNA-mediated knockdown or overexpressing the FAK CD can decrease cell proliferation and tumor growth in breast cancer cells (Golubovskaya et al. 2009). Collectively, these studies suggest that FAK is critical in cancer cell survival.
Regulation of Cancer Stem Cells
Cancer stem cells (CSCs) have the ability to self-renew and to differentiate into cancer cells from a rare population of undifferentiated tumorigenic cells (Patel and Chen 2012). CSCs were first isolated from leukemia (Bonnet and Dick 1997) and, later, from many solid tumors, including brain, breast, prostate and pancreas cancers (Al-Hajj et al. 2003; Li et al. 2007; Li et al. 2009; Patrawala et al. 2006; Singh et al. 2003). CSCs generally contain specific cell surface markers, such as CD133, CD44, CD90, and CD24 (Anido et al. 2010; Singh et al. 2003) in addition to expressing specific transcription factors (Liu et al. 2013). FAK deletion in a murine breast cancer model led to a decrease in the number of mammary CSCs and a decrease in their self-renewal potential; this ultimately inhibited tumor progression (Luo et al. 2009a). Recent studies have also indicated that FAK is involved in the expression of several stem cell factors. FAK maintains the expression of critical transcription factors Slug (Snail family zinc finger 2) and Sox9, which were identified as important factors in maintaining mammary CSCs (Guo et al. 2012). In addition, NANOG, a key marker in stem cells, increases the level of FAK expression and activity in 293, SW480, and SW620 cancer cells (Ho et al. 2012). NANOG directly binds to the FAK promoter triggering FAK expression, and studies show that downregulating NANOG expression by siRNA can inhibit cancer cell growth, which can be reversed by FAK overexpression (Ho et al. 2012). These studies indicate that FAK expression may have an important role in the control of CSC function and activity.
Epithelial-to-Mesenchymal Transition (EMT)
EMT is a crucial process during embryogenesis, development, tissue remodeling and tumor progression. Over the past decade, numerous regulators have been identified as essential transcription factors in EMT, such as Snail, Slug, Twist, and Zeb (Chui 2013; Wang et al. 2013). EMT ultimately requires a decrease in epithelial markers (E-cadherin, α-catenin, and β-catenin), an increase in mesenchymal markers (vimentin, fibronectin, and N-cadherin) and the secretion of matrix metalloproteinases (MMPs). These changes in cell phenotype and genetic modulation promote a transition from benign tumor to invasive carcinoma.
Recent studies have identified evidence of FAK involvement in the EMT process. FAK has a functional role in TGF-β-mediated EMT by Src-dependent activation in hepatocytes (Cicchini et al. 2008). These studies revealed that FAK signaling is required for the transcriptional regulation of several mesenchymal markers and for the delocalization of E-cadherin. Additionally, a FAK inhibitor (1,2,4,5-benzenentetramine, 4HCl) repressed TGF-β-induced EMT in human squamous cell carcinoma (Saito et al. 2013). FAK signaling was required for Src-regulated E-cadherin expression in colon cancer cells, and inhibition of FAK activity reduced Src-mediated cell invasion (Avizienyte et al. 2002; Hauck et al. 2002a). More direct evidence of FAK involvement in EMT has been provided from a recent study of FAK-/- embryonic cells. FAK re-expression rescued the mesenchymal characteristics of FAK-/- embryonic cells to generate committed mouse embryonic fibroblasts via Snail1 gene expression and Snail1 protein stabilization (Li et al. 2011). Taken together, although the direct role of FAK is yet to be unveiled in EMT, the correlation between FAK and EMT may offer an important target in cancer metastasis and cancer therapeutics.
Invasion and Metastasis
FAK overexpression is also associated with the enhanced invasion and metastatic characteristics of EMT (Cance et al. 2000a). Integrin β1 and FAK signaling directly regulate the proliferation and invasion of metastatic cells in the lung (Shibue and Weinberg 2009). FAK phosphorylation is important in regulating E-cadherin expression by activating Src signaling pathways in colon cancers and, before small molecule FAK inhibitors were available, overexpression of the FAK CD was useful in inhibiting cancer cell invasion and metastasis (Hauck et al. 2002b). Furthermore, FAK promotes cellular membrane expression of MT1-MMP, a matrix metalloproteinase, which serves to degrade the ECM (Wu et al. 2005). A recent study from Schlaepfer’s group highlighted the importance of FAK in the tumor environment. FAK plays an important role in mediating VEGF (vascular endothelial growth factor)-induced vascular permeability by directly phosphorylating β-catenin at Y142 (Chen et al. 2012). In addition, vascular endothelial cadherin (VEC) phosphorylation, which is mediated by FAK, increases vascular permeability and cancer cell metastasis (Jean et al. 2014). Cancer cells release copious amounts of VEGF, which compromises barrier function by increasing vascular permeability and new blood vessel formation. FAK-mediated barrier function demonstrates how cancer cells take advantage of the compromised endothelial barrier and enter the vasculature in intravasation and extravasation processes.
Tumor Angiogenesis
FAK also plays an important role in tumor angiogenesis. FAK expression is increased in microvascular endothelial cells, which leads to enhanced angiogenesis (Haskell et al. 2003; Lechertier and Hodivala-Dilke 2012). VEGF stimulation increases the activation of FAK in endothelial cells (Chen et al. 2012). Increased FAK Y397 autophosphorylation is also correlated with increased endothelial cell migration (Cary et al. 1996). In addition, inhibition of FAK reduces VEGF expression from tumor cells and has been shown to decrease tumor angiogenesis in the 4T1 breast cancer model (Mitra and Schlaepfer 2006). Since FAK has an important role in the regulation of tumor angiogenesis, blocking FAK activity in endothelial cells should be considered in cancer prevention therapy.
Pyk2: FAK Family Protein
Pyk2 (proline-rich tyrosine kinase 2) is the only other kinase structurally and functionally related to FAK, also referred to as the second FAK family kinase. FAK and Pyk2 contain approximately 45% homologous amino acid sequences over the entire protein, and even up to 60% homology within the kinase domain (Fig. 2) (Lipinski and Loftus 2010). Despite these similarities, these two proteins are not equally expressed in all types of tissues. Pyk2 expression, in contrast to FAK, is limited to brain cells, fibroblasts, platelets, and hematopoietic cell types (Avraham et al. 2000; Orr and Murphy-Ullrich 2004). FAK is primarily activated through integrins, growth factor receptors, and cytokine receptors in contact with the ECM, whereas Pyk2 can be activated by integrins, and shares downstream targets with FAK, but it is usually activated in response to increases in intracellular calcium (Lev et al. 1995).
FAK is typically overexpressed in various cancers, and Pyk2 is also upregulated in several cancers (Wendt et al. 2013; Zhonghua et al. 2012). In breast cancer cells, Pyk2 overexpression was inversely related to E-cadherin level and necessary to initiate metastasis (Wendt et al. 2013). In benign prostatic hyperplasia, Pyk2 overexpression was observed and resulted in increased malignancy in prostate cancer (Stanzione et al. 2001). Interestingly, compensatory Pyk2 upregulation is often observed when FAK is deleted or inhibited. Pyk2 is upregulated in FAK-/- fibroblasts and endothelial cell-specific conditional FAK knock-out mice (Sieg et al. 1998; Weis et al. 2008). In adult mice lacking endothelial cell FAK, tumor angiogenesis can be promoted by compensatory Pyk2 upregulation (Weis et al. 2008), suggesting that Pyk2 plays an important role in tumor angiogenesis. Taken together, these two kinases may have overlapping signaling pathways in cancer progression, and it would be ideal to inhibit both FAK and Pyk2 activity.
FAK Inhibitors
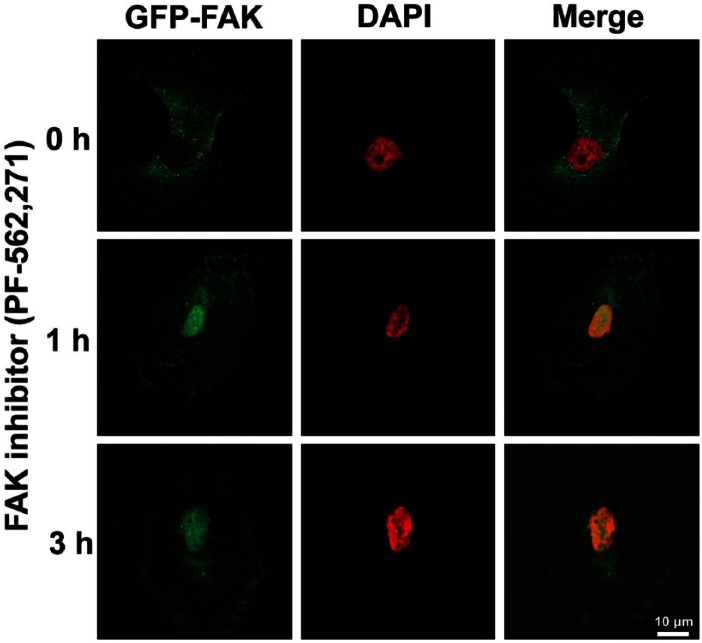
As FAK expression and activity play important roles in tumor survival, motility, and angiogenesis in vitro and in vivo, FAK is recognized as a potential cancer target. Therefore, there have been numerous efforts to inhibit FAK signaling in cancer therapy. FAK inhibitors have changed significantly since the introduction of inhibiting FAK activity via antisense cDNA (Xu et al. 1996) and expression of the FAK CD (Xu et al. 2000). Major pharmaceutical companies have developed pharmacological FAK inhibitors, and some of these—mostly small molecule inhibitors and small inhibitory peptides—have made it to clinical trials (Table 2). The small molecule FAK inhibitors are ATP analogs in either pyrimidine- or pyridine-base form. FAK inhibitors can be mainly used to inhibit FAK activity in cancer cells by direct inhibition of FAK kinase activity. Our recent study demonstrated that treatment with the small molecule FAK inhibitor (PF-562,271) inhibited FAK activities as well as enhanced FAK nuclear localization in RPMI-7951 human melanoma (Fig. 3; unpublished data), suggesting that FAK inhibition also promotes FAK kinase-independent function (see also Fig. 1) via nuclear FAK localization, which may inhibit tumor growth and metastasis.
Table 2.
Pharmacological FAK Inhibitors.
| Inhibitor | Company | Type and Target | Clinical Trial* | Identifiers/References |
|---|---|---|---|---|
| TAE226 | Novartis | ATP-competitive kinase inhibitor; FAK and Pyk2 | Preclinical | None/(Halder et al. 2007; Liu et al. 2007) |
| PF-573,228 | Pfizer | ATP-competitive kinase inhibitor; FAK | Preclinical | None/(Slack-Davis et al. 2007) |
| GSK-2256098 | GlaxoSmithKline | ATP-competitive kinase inhibitor; FAK | Phase I | NCT01138033 and NCT00996671/(Bottsford-Miller 2011) |
| PND-1186 (VS-4718) | Poniard (Verastem) | ATP-competitive kinase inhibitor; FAK | Phase I | NCT01849744/(Tanjoni et al. 2010; Walsh et al. 2010) |
| PF-562,271 (VS-6062) | Pfizer (Verastem) | ATP-competitive kinase inhibitor; FAK and Pyk2 | Phase I | NCT00666926/(Shapiro et al. 2014; Stokes et al. 2011) |
| PF-04554878 (VS-6063) | Pfizer (Verastem) | Unknown | Phase II | NCT02004028 and NCT01778803/None |
| Y15 | Cure FAKtor | Protein scaffold inhibitor; FAK | Preclinical | None/(Golubovskaya et al. 2012) |
| Y11 | Cure FAKtor | Protein scaffold inhibitor; FAK | Preclinical | None/(Hochwald et al. 2009) |
Clinical trial identifier is available at ClinicalTrials.gov.
Figure 3.
Pharmacological FAK inhibition promotes nuclear localization of FAK in RPMI-7951 human melanoma cells. GFP-FAK is stably expressed in RPMI-7951 cells. Cells were treated with PF-562,271 (1 μM) for 1–3 hr and cells were fixed. Images were collected with Nikon confocal microscope (A1R) and analyzed with NIS-Elements AR software. Nuclear localized FAK can be observed within 1 hr treatment of the FAK inhibitor. Green, FAK; Pseudo-colored Red, DAPI. Scale, 10 µm.
TAE-226
A majority of the available FAK inhibitors work as an ATP analog and target the ATP-binding domain surrounding lysine 454 in the FAK kinase domain. TAE-226 (Novartis) was the first effective FAK inhibitor used in vitro and in vivo. TAE-226 has been shown to inhibit FAK kinase activity as well as that of Pyk2 and other protein tyrosine kinases including insulin-like growth factor-1 receptor (Halder et al. 2007). TAE-226 blocks cell proliferation and invasion, and increases apoptosis in glioma and ovarian tumor models (Halder et al. 2007; Liu et al. 2007; Shi et al. 2007). Interestingly, TAE-226 in combination with docetaxel, a microtubule stabilizer, significantly decreases angiogenesis and invasion in ovarian carcinoma (Halder et al. 2007).
PF-573,228
PF-573,228 (Pfizer) is an ATP analog that acts to inhibit FAK kinase activity. This inhibitor is highly specific for FAK catalytic activity and other related kinases. Treatment with PF-573,228 at its IC50, 4.0 nM, blocks FAK kinase activity without affecting Pyk2 activity (Slack-Davis et al. 2007). Although PF-573,228 inhibits tumor cell migration in vitro, it cannot inhibit cell proliferation or induce apoptosis in fibroblasts and in prostate cancer tissues (Slack-Davis et al. 2007). These results suggested that PF-573,228 may have a different mode of action for cancer treatment and may effectively target FAK motility or metastasis in tumors.
GSK2256098
GSK2256098 (GlaxoSmithKline) is an orally available FAK inhibitor and was tested in a Phase I study (clinical trial identifier at ClinicalTrials.gov; NCT00996671). GSK2256098 treatment significantly reduced Y397 autophosphorylation of FAK at 1.0 μM (not for PYK2) and decreased migration and invasion in SKOV3 ovarian cancer cell line (Bottsford-Miller 2011). In addition, the 75 mg/kg dose was shown to significantly decrease tumor volume by 58% in a mouse xenograft model (Bottsford-Miller 2011). In this study, the double and triple combination of GSK2256098 with cytotoxic drugs including pazopanib, a multi-targeted receptor tyrosine kinase inhibitor, and docetaxel, a microtubule stabilizer, resulted in a 99% reduction in tumor weight.
VS-4718 (Previously PND-1186, Poniard)
VS-4718 (Verastem) effectively blocks both FAK and Pyk2 activity, and is currently in Phase I trials against various tumors (Clinical trial identifier; NCT01849744). Preclinical studies have demonstrated that VS-4718 has a high anti-tumor efficacy in a breast carcinoma mouse model (Tanjoni et al. 2010). Interestingly, VS- 4718 treatment was shown to reduce inflammatory cell infiltration and IL-6 expression, suggesting that FAK inhibition is associated with anti-inflammatory action in both tumor and stromal cells (Walsh et al. 2010). In addition, the oral administration of VS-4718 inhibited tumor growth through caspase-3 activation, and decreased tumor experimental metastasis in ovarian carcinoma models (Tancioni et al. 2014).
VS-6062 (Previously PF-562,271, Pfizer)
VS-6062 (Verastem) is capable of inhibiting both FAK and Pyk2 kinase activity (Roberts et al. 2008). VS-6062 is very effective both in vitro and in vivo by reducing tumor size and preventing metastasis (Stokes et al. 2011). In angiogenesis, compensatory Pyk2 upregulation in endothelial cells was observed upon inhibition or ablation of FAK (Weis et al. 2008). Therefore, the dual inhibition of FAK and Pyk2 is beneficial. In this respect, VS-6062 was shown to reduce human epithelial ovarian cancer angiogenesis and metastasis in nude mice (Stone et al. 2014). VS-6062 was actually the first FAK inhibitor tested in clinical Phase I trials (Infante et al. 2012), which established the recommended dosage of VS-6062, and overall showed that approximately 34% of patients experienced stable disease state at the end of the six-week treatment. However, VS-6062 showed non-linear pharmacokinetics and was thus discontinued (Infante et al. 2012).
VS-6063 (Previously PF-04554878, Pfizer)
VS-6063 (Verastem) was originally developed by Pfizer to overcome the nonlinear pharmacokinetics of VS-6062/PF-562,271. The results of a Phase I trial of VS-6063 indicated that stability was achieved in some patients with ovarian and colorectal tumors (clinical trial identifier; NCT017788033). VS-6063 is currently in Phase II study in patients with KRAS mutant non-small cell lung cancer (clinical trial identifier; NCT01951690). Although the chemical structure and specificity of VS-6063 are still not published, VS-6063 treatment has been shown to increase ovarian carcinoma cell sensitivity to taxane, suggesting that VS-6063 in combination with paclitaxel may be more effective as compared with monotherapy (clinical trial identifier; NCT017788033).
Y15 and Y11
There are drawbacks with using FAK inhibitors that function as ATP analogs to target the ATP binding pocket of the FAK kinase domain. Many tyrosine kinases share conserved sequences in their ATP binding sites, and it increases the probability that these FAK inhibitors could bind to and inhibit other kinases, diminishing the specificity of the ATP analogs to FAK (Golubovskaya et al. 2012). A new way to avoid this problem is to directly interfere with FAK autophosphorylation. This will not only inhibit FAK activity but also decrease the probability of inhibiting other tyrosine kinases.
Both 1,2,4,5-Benzenetetraamine tetrahydrocloride (FAK Inhibitor 14 or Y15) and 1-(2-Hydroxyethyl)-3,5,7-triaza-1-azaniatricyclo[3.3.1.13,7] decane bromide (Y11) are small molecule inhibitors of FAK but are not ATP analogs. These two molecules act by binding to and inhibiting the autophosphorylation at FAK Y397 (Golubovskaya et al. 2012; Golubovskaya et al. 2008). Y15 and Y11 have been shown to inhibit cell viability in breast, colon, melanoma, lung and pancreatic cancer cell lines (Golubovskaya et al. 2012; Golubovskaya et al. 2008; Hochwald et al. 2009). Y15 has an IC50 of about 1 μM, whereas Y11 has an IC50 of about 50 nM. Treatment with 1 μM Y15 or Y11 selectively inhibits FAK activity without interfering with the activity of other kinases (Golubovskaya et al. 2012; Golubovskaya et al. 2008). These two FAK inhibitors offer the potential for obtaining greater selectivity by targeting Y397 instead of the ATP binding site.
FAK Genetic Animal Models
The first genetic FAK mouse generated was a global ablation model (Ilic et al. 1995). These studies revealed that FAK deletion caused embryonic lethality at embryonic day E8.5 due to defects in mesodermal development, specifically cardiovascular development, suggesting that FAK is essential in embryonic vasculogenesis. Another mouse model with homologous knock-in mutations at FAK lysine 454 to arginine 454 (termed kinase-dead, KD), the ATP-binding residue, demonstrated that mice were lethal at E9.5 because of defects in vasculogenesis and chorio-allantois defects (Lim et al. 2010). Furthermore, another mutant FAK mouse model was developed with the deletion of exon 15, which contains autophosphorylation site Y397 (Corsi et al. 2009). These mice developed normally at E12.5, but died at E14.5-E16.5, with multiple developmental defects including hemorrhages, edema, delayed artery formation, vascular remodeling defects, and multiple organ abnormalities. This suggests that FAK has important kinase roles independent of autophosphorylation activity in early development. To avoid an embryonic lethal phenotype of various global FAK mutations in mice, several mouse models have utilized the inducible Cre-Lox recombinase genetic system to induce conditional FAK knockouts during development or in adult mice (Table 3).
Table 3.
FAKflox/flox Knockout and FAK Kinase-Dead (KD) Knock-in Mouse Models.
| Type of Cell | Promoter | Phenotype | References |
|---|---|---|---|
| Embryonic vascular ECs | Tie2 | Developmental vasculature defects | (Braren et al. 2006; Shen et al. 2005) |
| Adult vascular ECs | SCL, PDGF-B | Impact on tumor angiogenesis and sensitization tumor cell death by chemotherapy | (Schmidt et al. 2013; Tavora et al. 2010; Tavora et al. 2014; Weis et al. 2008) |
| Keratinocytes | K5, K14 | Inhibition of malignant skin tumor progression | (McLean et al. 2004b; Serrels et al. 2012) |
| Mammary ECs | MMTV | Inhibition of breast tumor formation and metastasis | (Luo et al. 2009b; Nagy et al. 2007) |
| Prostate cells | Probasin | Decreased prostate carcinoma | (Slack-Davis et al. 2009) |
| FAK-KD knockin in ECs (FAKflox/KD) | SCL, Tie2 | Reduced vascular permeability and inhibition of tumor metastasis | (Jean et al. 2014; Zhao et al. 2010) |
SCL, stem cell leukemia; PDGF-B, platelet-derived growth factor-B; K5 and 14, keratin-5 and -14; MMTV, mouse mammary tumor virus.
Endothelial Cells
Two independent groups generated Tie2-Cre driven endothelial cell knockout mice and showed that embryos died at E11.5 with developmental vasculature defects (Braren et al. 2006; Shen et al. 2005). A tamoxifen-inducible endothelial cell-specific knockout mouse (SCL-Cre-ER(T)) study revealed that FAK knockout in adult mice endothelial cells was not lethal. Surprisingly, there were no major tumor angiogenesis defects due to compensatory Pyk2 expression upon the loss of FAK (Weis et al. 2008). This result suggests the need for a dual protein tyrosine kinase inhibitor that targets FAK and Pyk2 so as to provide a total anti-angiogenic effect. To avoid Pyk2 upregulation in genetic FAK knockout mice, endothelial cell-specific, kinase-dead mice were generated by crossing FAKflox/flox SCL-Cre-ER(T) and FAK WT/KD mice. These mice exhibited defects in VEGF-mediated endothelial permeability by deregulation of adherens junctions (Chen et al. 2010; Jean et al. 2014).
Endothelial cell-specific FAK deletion with a tamoxifen-inducible platelet-derived growth factor-B (PDGF-B) Cre mouse demonstrated that, in contrast to the results shown from previous EC FAK knockout mouse models, FAK deletion in adult mice inhibited tumor growth and reduced angiogenesis (Tavora et al. 2010). With the same mouse model, cancer cells were injected subcutaneously and allowed to grow for just over 7 days. Mice were then injected with tamoxifen and given a tamoxifen-containing diet to delete endothelial cell FAK. These mice then underwent chemotherapy with doxorubicin or irradiation. Surprisingly, endothelial cell FAK knockout sensitized the tumors to chemotherapy (Tavora et al. 2014), suggesting that EC FAK may play a novel role in protecting tumors from chemotherapy, and that targeting EC FAK may enhance current cancer therapeutics.
Keratinocytes
Mice with a floxed FAK kinase domain under the control of tamoxifen-inducible keratin-14 Cre (K14 CreER(T)) were generated. Skin tumors were induced chemically and the loss of FAK inhibited tumor formation (McLean et al. 2004a). FAK-deleted squamous skin carcinoma cells (SCCs) were obtained from K14 CreER(T) FAKflox/flox mice and FAK re-expression in SCCs restored tumor-forming activity. But, importantly, PF-562,271 treatment abolished tumor proliferation. This result suggests that kinase activity of FAK is required for skin tumor progression in this system (Serrels et al. 2012).
Mammary Epithelial Cells
To evaluate the importance of FAK in mammary tumorigenesis, several groups have generated MMTV (mouse mammary tumor virus)-Cre mice, interbred from conditional FAK flox mice (Lahlou et al. 2007; Luo et al. 2009a; Provenzano et al. 2008). The first FAK-deleted mice using this system resulted in an inhibition of the benign-to-malignant transformation of tumors as well as inhibition of mammary tumor cell proliferation. This provided evidence that FAK plays an important role in initiating mammary tumorigenesis (Lahlou et al. 2007). Another conditional FAK knockout in mammary epithelium limited breast tumor formation and metastasis (Provenzano et al. 2008). In MMTV-Cre mice with a conditional deletion of FAK exon 3, it was demonstrated that FAK deletion in the mammary epithelium limits tumor formation via its effect on mammary cancer stem and progenitor cells (Luo et al. 2013).
Prostate Cells
Transgenic adenocarcinoma of the mouse prostate is a well-studied mouse model of prostate cancer under the control of the androgen-responsive minimal rat probasin promoter (Greenberg et al. 1995; Kaplan-Lefko et al. 2003). In this mouse model, the loss of FAK using probastin-Cre expression or a pharmacological FAK inhibitor, PF-562,271, did not affect the progression of the adenocarcinoma. However, continued FAK expression and activity is needed for androgen-independent formation of neuroendocrine carcinomas (Slack-Davis et al. 2009).
Nuclear FAK in Cancer
In addition to the canonical roles of FAK in the regulation of development, migration, proliferation, tumor angiogenesis, and metastasis, several recent studies have identified novel roles for FAK in the nucleus (Fig. 1). One of these studies identified that nuclear FAK promotes cell proliferation and survival by reducing p53-mediated cell cycle arrest under stress conditions (Lim et al. 2008b). Genetic FAK inhibition (kinase-dead knock-in cells) or pharmacological FAK inhibition increased FAK accumulation in the nucleus, which prevented inflammatory VCAM-1 expression by promoting the degradation of a transcription factor, specifically GATA4, which is required for VCAM-1 expression (Lim et al. 2012). Collectively, there are several conditions that can stimulate FAK nuclear localization: 1) stress signals, 2) cell de-adhesion, 3) FAK inhibition (Lim et al. 2008b; Lim et al. 2012). However, the mechanisms by which FAK shuttles from the membrane to the nucleus remain undefined (Lim 2013; Lim et al. 2008b).
In human melanoma cells, we found that FAK inhibition with PF-562,271 not only inhibits FAK activity, but also enhances FAK nuclear localization (Fig. 3).
Taken together, these studies have provided evidence of how nuclear FAK could regulate cancer cell proliferation and motility. Current evidence indicates that nuclear FAK may provide a potential mechanism for cancer therapeutics.
FAK Regulation of Epigenetics
Recently, it was reported that nuclear FAK binds to methyl CpG-binding protein 2 (MBD2) in myoblast cells, and their binding enhanced heterochromatin remodeling to promote gene expression (Luo et al. 2009c). The nuclear FAK-MBD2 complex decreased histone deacetylase complex 1 (HDAC1) and the methyl CpG site in the myogenin promoter, resulting in myogenin expression (Luo et al. 2009c). In addition to the known role of nuclear FAK, which controls transcription factor stability via ubiquitination, these studies suggest that FAK may play an important role in the regulation of gene expression through interactions with molecules responsible for DNA modification.
Conclusions
Preclinical and clinical trials of small molecule FAK inhibitors have demonstrated that FAK can be an effective cancer target in various cancers. Interestingly, based on genetic FAK mouse studies in cancer progression, we now know that FAK plays critical roles in promoting invasive properties of tumor cells as well as the tumor environment, which controls tumor metastasis. Therefore, FAK inhibitors have beneficial effects in terms of blocking tumor progression in both tumor cells and tumor stroma.
An emerging role of FAK signaling is the dual ability of FAK inhibition to inhibit FAK activity and promote nuclear FAK to potentially regulate gene expression. As FAK moves to the nucleus upon FAK inhibition via genetic or pharmacological means, it will be extremely intriguing to explore as yet unknown functions of nuclear FAK and reveal unidentified targets of FAK inhibitors for cancer therapy.
Acknowledgments
We thank Dr. Erin Ahn for critical reading of the manuscript.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by 2013-2015 Abraham Mitchell Cancer Research fund 298036 to S.L. and American Heart Association National Scientist Development Grant 12SDG10970000 to S.L.
References
- Agochiya M, Brunton VG, Owens DW, Parkinson EK, Paraskeva C, Keith WN, Frame MC. (1999). Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene 18:5646-5653. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. (2003). Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100:3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, Cuartas I, Raventos C, Martinez-Ricarte F, Poca MA, Garcia-Dorado D, Lahn MM, Yingling JM, Rodon J, Sahuquillo J, Baselga J, Seoane J. (2010). TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell 18:655-668. [DOI] [PubMed] [Google Scholar]
- Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. (2002). Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol 4:632-638. [DOI] [PubMed] [Google Scholar]
- Avraham H, Park SY, Schinkmann K, Avraham S. (2000). RAFTK/Pyk2-mediated cellular signalling. Cell Signal 12:123-133. [DOI] [PubMed] [Google Scholar]
- Barsukov IL, Prescot A, Bate N, Patel B, Floyd DN, Bhanji N, Bagshaw CR, Letinic K, Di Paolo G, De Camilli P, Roberts GC, Critchley DR. (2003). Phosphatidylinositol phosphate kinase type 1gamma and beta1-integrin cytoplasmic domain bind to the same region in the talin FERM domain. J Biol Chem 278:31202-31209. [DOI] [PubMed] [Google Scholar]
- Beierle EA, Massoll NA, Hartwich J, Kurenova EV, Golubovskaya VM, Cance WG, McGrady P, London WB. (2008). Focal adhesion kinase expression in human neuroblastoma: immunohistochemical and real-time PCR analyses. Clin Cancer Res 14:3299-3305. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730-737. [DOI] [PubMed] [Google Scholar]
- Bottsford-Miller J, Sanguino A., Duangmani Thanapprapasr D., Pecot C.V., Stone R.L., Auger K., Nick A.M., Sood A.K. (2011) Enhancing anti-angiogenic therapy by blocking focal adhesion kinase In Cancer Res. 230 [Google Scholar]
- Brami-Cherrier K, Gervasi N, Arsenieva D, Walkiewicz K, Boutterin MC, Ortega A, Leonard PG, Seantier B, Gasmi L, Bouceba T, Kadare G, Girault JA, Arold ST. (2014). FAK dimerization controls its kinase-dependent functions at focal adhesions. EMBO J 33:356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. (2006). Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol 172:151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb M, Polte T, Hanks SK. (1995). Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for the Src family kinases. Mol Cell Biol 15:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance WG, Golubovskaya VM. (2008). Focal adhesion kinase versus p53: apoptosis or survival? Sci Signal 1:pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. (2000a). Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 6:2417-2423. [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. (2000b). Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 6:2417-2423. [PubMed] [Google Scholar]
- Canel M, Secades P, Rodrigo JP, Cabanillas R, Herrero A, Suarez C, Chiara MD. (2006). Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. Clin Cancer Res 12:3272-3279. [DOI] [PubMed] [Google Scholar]
- Cary LA, Chang JF, Guan J-L. (1996). Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci 108:1787-1794. [DOI] [PubMed] [Google Scholar]
- Ceccarelli DF, Song HK, Poy F, Schaller MD, Eck MJ. (2006). Crystal structure of the FERM domain of focal adhesion kinase. J Biol Chem 281:252-259. [DOI] [PubMed] [Google Scholar]
- Chan PC, Lai JF, Cheng CH, Tang MJ, Chiu CC, Chen HC. (1999). Suppression of ultraviolet irradiation-induced apoptosis by overexpression of focal adhesion kinase in Madin-Darby canine kidney cells. J Biol Chem 274:26901-26906. [DOI] [PubMed] [Google Scholar]
- Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD. (2012). VEGF-Induced Vascular Permeability Is Mediated by FAK. Developmental cell 22:146-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Guo L, Sprang SR, Sternweis PC. (2011). Modulation of a GEF switch: autoinhibition of the intrinsic guanine nucleotide exchange activity of p115-RhoGEF. Protein science : a publication of the Protein Society 20:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Medina F, Liu MY, Thomas C, Sprang SR, Sternweis PC. (2010). Activated RhoA binds to the pleckstrin homology (PH) domain of PDZ-RhoGEF, a potential site for autoregulation. The Journal of biological chemistry 285:21070-21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui MH. (2013). Insights into cancer metastasis from a clinicopathologic perspective: Epithelial-Mesenchymal Transition is not a necessary step. Int J Cancer 132:1487-1495. [DOI] [PubMed] [Google Scholar]
- Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. (2008). TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res 314:143-152. [DOI] [PubMed] [Google Scholar]
- Cooper LA, Shen TL, Guan JL. (2003). Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol 23:8030-8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi JM, Houbron C, Billuart P, Brunet I, Bouvree K, Eichmann A, Girault JA, Enslen H. (2009). Autophosphorylation-independent and dependent functions of Focal Adhesion Kinase during development. J Biol Chem 284:34769-34776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi JM, Rouer E, Girault JA, Enslen H. (2006). Organization and post-transcriptional processing of focal adhesion kinase gene. BMC Genomics 7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XQ, Liu XF, Yao L, Chen CQ, Gu ZD, Ni PH, Zheng XM, Fan QS. (2014). Somatic mutational analysis of FAK in breast cancer: a novel gain-of-function mutation due to deletion of exon 33. Biochem Biophys Res Commun 443:363-369. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. (2010). Cell mechanics and the cytoskeleton. Nature 463:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. (2010). The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol 11:802-814. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. (1996). Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 134:793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Doi R, Mori T, Toyoda E, Ito D, Kami K, Koizumi M, Kida A, Kawaguchi Y, Fujimoto K. (2006). Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg 30:219-226. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Figel S, Ho BT, Johnson CP, Yemma M, Huang G, Zheng M, Nyberg C, Magis A, Ostrov DA, Gelman IH, Cance WG. (2012). A small molecule focal adhesion kinase (FAK) inhibitor, targeting Y397 site: 1-(2-hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo [3.3.1.1(3,7)]decane; bromide effectively inhibits FAK autophosphorylation activity and decreases cancer cell viability, clonogenicity and tumor growth in vivo. Carcinogenesis 33:1004-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Finch R, Cance WG. (2005). Direct Interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J Biol Chem 280:25008-25021. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, Cance WG. (2008). A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem 51:7405-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Zheng M, Zhang L, Li JL, Cance WG. (2009). The direct effect of focal adhesion kinase (FAK), dominant-negative FAK, FAK-CD and FAK siRNA on gene expression and human MCF-7 breast cancer cell tumorigenesis. BMC Cancer 9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. (1995). Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A 92:3439-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zurrer-Hardi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, Weinberg RA. (2012). Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148:1015-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann W, Fernandez A, Lopez-Berestein G, Sood AK. (2007). Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res 67:10976-10983. [DOI] [PubMed] [Google Scholar]
- Haskell H, Natarajan M, Hecker TP, Ding Q, Stewart J, Jr., Grammer JR, Gladson CL. (2003). Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin Cancer Res 9:2157-2165. [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Ilic D, Schlaepfer DD. (2002a). v-Src SH3-enhanced interaction with focal adhesion kinase at beta 1 integrin-containing invadopodia promotes cell invasion. J Biol Chem 277:12487-12490. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Schlaepfer DD. (2002b). The focal adhesion kinase - a regulator of cell migration and invasion. IUBMB Life 53:115-119. [DOI] [PubMed] [Google Scholar]
- He ZX, He HW, Wang D, Fang MX. (2006). [Expression and clinical significance of focal adhesion kinase in oral squamous cell carcinoma]. Sichuan Da Xue Xue Bao Yi Xue Ban 37:876-878. [PubMed] [Google Scholar]
- Ho B, Olson G, Figel S, Gelman I, Cance WG, Golubovskaya VM. (2012). Nanog increases focal adhesion kinase (FAK) promoter activity and expression and directly binds to FAK protein to be phosphorylated. J Biol Chem 287:18656-18673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, Magis A, Ostrov D, Cance WG, Golubovskaya VM. (2009). A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle 8:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN. (1995). The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol 60:1-35. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. (1995). Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, Fingert H, Pierce KJ, Xu H, Roberts WG, Shreeve SM, Burris HA, Siu LL. (2012). Safety, Pharmacokinetic, and Pharmacodynamic Phase I Dose-Escalation Trial of PF-00562271, an Inhibitor of Focal Adhesion Kinase, in Advanced Solid Tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 30:1527-1533. [DOI] [PubMed] [Google Scholar]
- Jean C, Chen XL, Nam JO, Tancioni I, Uryu S, Lawson C, Ward KK, Walsh CT, Miller NL, Ghassemian M, Turowski P, Dejana E, Weis S, Cheresh DA, Schlaepfer DD. (2014). Inhibition of endothelial FAK activity prevents tumor metastasis by enhancing barrier function. J Cell Biol 204:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, McCarty JH. (2012). Band 4.1 proteins regulate integrin-dependent cell spreading. Biochem Biophys Res Commun 426:578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. (2003). Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 55:219-237. [DOI] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ. (2007). Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A 104:20302-20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, Cance W. (2005). High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol 18:1289-1294. [DOI] [PubMed] [Google Scholar]
- Lechertier T, Hodivala-Dilke K. (2012). Focal adhesion kinase and tumour angiogenesis. J Pathol 226:404-412. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. (1995). Protein tyrosine kinase PYK2 involved in calcium-induced regulation of ion channel and MAP kinase functions. Nature 376:737-745. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. (2007). Identification of pancreatic cancer stem cells. Cancer Res 67:1030-1037. [DOI] [PubMed] [Google Scholar]
- Li C, Lee CJ, Simeone DM. (2009). Identification of human pancreatic cancer stem cells. Methods Mol Biol 568:161-173. [DOI] [PubMed] [Google Scholar]
- Li XY, Zhou X, Rowe RG, Hu Y, Schlaepfer DD, Ilic D, Dressler G, Park A, Guan JL, Weiss SJ. (2011). Snail1 controls epithelial-mesenchymal lineage commitment in focal adhesion kinase-null embryonic cells. J Cell Biol 195:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. (2007). Structural basis for the autoinhibition of focal adhesion kinase. Cell 129:1177-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-T, Mikolon D, Stupack DG, Schlaepfer DD. (2008a). FERM control of FAK function: Implications for cancer therapy. Cell Cycle 7:2306-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST. (2013). Nuclear FAK: a new mode of gene regulation from cellular adhesions. Mol Cells 36:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. (2008b). Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell 29:9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Tomar A, Miller NL, Yoo J, Schlaepfer DD. (2010). Knock-in mutation reveals an essential role for focal adhesion kinase activity in blood vessel morphogenesis and cell motility-polarity but not cell proliferation. J Biol Chem 285:21526-21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Miller NL, Chen XL, Tancioni I, Walsh CT, Lawson C, Uryu S, Weis SM, Cheresh DA, Schlaepfer DD. (2012). Nuclear-localized focal adhesion kinase regulates inflammatory VCAM-1 expression. The Journal of cell biology 197:907-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski CA, Loftus JC. (2010). Targeting Pyk2 for therapeutic intervention. Expert Opin Ther Targets 14:95-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Yu X, Liu S. (2013). Pluripotency transcription factors and cancer stem cells: small genes make a big difference. Chin J Cancer 32:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TJ, LaFortune T, Honda T, Ohmori O, Hatakeyama S, Meyer T, Jackson D, de Groot J, Yung WK. (2007). Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther 6:1357-1367. [DOI] [PubMed] [Google Scholar]
- Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S, Wicha MS, Guan JL. (2009a). Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res 69:466-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Zhao X, Chen S, Liu S, Wicha MS, Guan JL. (2013). Distinct FAK activities determine progenitor and mammary stem cell characteristics. Cancer Res 73:5591-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SW, Zhang C, Zhang B, Kim CH, Qiu YZ, Du QS, Mei L, Xiong WC. (2009b). Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. EMBO J 28:2568-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SW, Zhang C, Zhang B, Kim CH, Qiu YZ, Du QS, Mei L, Xiong WC. (2009c). Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. The EMBO journal 28:2568-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, Frame MC. (2004a). Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev 18:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, Frame MC. (2004b). Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev 18:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. (2005). Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56-68. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18:516-523. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K, Kuwano H. (2003). FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer 89:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy T, Wei H, Shen TL, Peng X, Liang CC, Gan B, Guan JL. (2007). Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J Biol Chem 282:31766-31776. [DOI] [PubMed] [Google Scholar]
- Ocak S, Chen H, Callison C, Gonzalez AL, Massion PP. (2012). Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer 118:1293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. (2004). Regulation of endothelial cell function BY FAK and PYK2. Front Biosci 9:1254-1266. [DOI] [PubMed] [Google Scholar]
- Owen JD, Ruest PJ, Fry DW, Hanks SK. (1999). Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol Cell Biol 19:4806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee BL, Yoon J, Kim J, Kim MA, Yang HK, Kim WH. (2010). Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum Pathol 41:1664-1673. [DOI] [PubMed] [Google Scholar]
- Parsons JT. (2003). Focal adhesion kinase: the first ten years. J Cell Sci 116:1409-1416. [DOI] [PubMed] [Google Scholar]
- Patel P, Chen EI. (2012). Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol (Lausanne) 3:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. (2006). Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25:1696-1708. [DOI] [PubMed] [Google Scholar]
- Peng X, Guan JL. (2011). Focal adhesion kinase: from in vitro studies to functional analyses in vivo. Curr Protein Pept Sci 12:52-67. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Beggs HE, Keely PJ. (2008). Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am J Pathol 173:1551-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. (2008). Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res 68:1935-1944. [DOI] [PubMed] [Google Scholar]
- Saito D, Kyakumoto S, Chosa N, Ibi M, Takahashi N, Okubo N, Sawada S, Ishisaki A, Kamo M. (2013). Transforming growth factor-beta1 induces epithelial-mesenchymal transition and integrin alpha3beta1-mediated cell migration of HSC-4 human squamous cell carcinoma cells through Slug. J Biochem 153:303-315. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. (1994). Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 14:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. (1998). Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: Summation of both c-Src and FAK-initiated tyrosine phosphorylation events. Mol Cell Biol 18:2571-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TT, Tauseef M, Yue L, Bonini MG, Gothert J, Shen TL, Guan JL, Predescu S, Sadikot R, Mehta D. (2013). Conditional deletion of FAK in mice endothelium disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities. Am J Physiol Lung Cell Mol Physiol 305:L291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels A, McLeod K, Canel M, Kinnaird A, Graham K, Frame MC, Brunton VG. (2012). The role of focal adhesion kinase catalytic activity on the proliferation and migration of squamous cell carcinoma cells. Int J Cancer 131:287-297. [DOI] [PubMed] [Google Scholar]
- Shapiro IM, Kolev VN, Vidal CM, Kadariya Y, Ring JE, Wright Q, Weaver DT, Menges C, Padval M, McClatchey AI, Xu Q, Testa JR, Pachter JA. (2014). Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med 6:237ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, Flavell RA, Gu H, Guan JL. (2005). Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol 169:941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Hjelmeland AB, Keir ST, Song L, Wickman S, Jackson D, Ohmori O, Bigner DD, Friedman HS, Rich JN. (2007). A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog 46:488-496. [DOI] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A 106:10290-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. (2000). FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2:249-256. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. (1998). Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J 17:5933-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821-5828. [PubMed] [Google Scholar]
- Slack-Davis JK, Hershey ED, Theodorescu D, Frierson HF, Parsons JT. (2009). Differential requirement for focal adhesion kinase signaling in cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Ther 8:2470-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. (2007). Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem 282:14845-14852. [DOI] [PubMed] [Google Scholar]
- Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ. (2004). Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol 165:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzione R, Picascia A, Chieffi P, Imbimbo C, Palmieri A, Mirone V, Staibano S, Franco R, De Rosa G, Schlessinger J, Tramontano D. (2001). Variations of proline-rich kinase Pyk2 expression correlate with prostate cancer progression. Lab Invest 81:51-59. [DOI] [PubMed] [Google Scholar]
- Stokes JB, Adair SJ, Slack-Davis JK, Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton AH, Hwang RF, Stelow EB, Parsons JT, Bauer TW. (2011). Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Molecular cancer therapeutics 10:2135-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RL, Baggerly KA, Armaiz-Pena GN, Kang Y, Sanguino AM, Thanapprapasr D, Dalton HJ, Bottsford-Miller J, Zand B, Akbani R, Diao L, Nick AM, DeGeest K, Lopez-Berestein G, Coleman RL, Lutgendorf S, Sood AK. (2014). Focal adhesion kinase: an alternative focus for anti-angiogenesis therapy in ovarian cancer. Cancer Biol Ther 15:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzmaier FJ, Jean C, Schlaepfer DD. (2014). FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14:598-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancioni I, Uryu S, Sulzmaier FJ, Shah NR, Lawson C, Miller NL, Jean C, Chen XL, Ward KK, Schlaepfer DD. (2014). FAK Inhibition Disrupts a beta5 Integrin Signaling Axis Controlling Anchorage-Independent Ovarian Carcinoma Growth. Mol Cancer Ther 13:2050-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjoni I, Walsh C, Uryu S, Nam JO, Mielgo A, Tomar A, Lim ST, Liang C, Koenig M, Sun C, Kwok C, Patel N, McMahon G, Stupack DG, Schlaepfer DD. (2010). PND-1186 FAK inhibitor selectively promotes tumor cell apoptosis in three-dimensional environments. Cancer Biology & Therapy 9:762-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavora B, Batista S, Reynolds LE, Jadeja S, Robinson S, Kostourou V, Hart I, Fruttiger M, Parsons M, Hodivala-Dilke KM. (2010). Endothelial FAK is required for tumour angiogenesis. EMBO Mol Med 2:516-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavora B, Reynolds LE, Batista S, Demircioglu F, Fernandez I, Lechertier T, Lees DM, Wong PP, Alexopoulou A, Elia G, Clear A, Ledoux A, Hunter J, Perkins N, Gribben JG, Hodivala-Dilke KM. (2014). Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature 514:112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis SE, Kouraklis GP, Kakisis JD, Kanelli HG, Apostolakou FE, Karatzas GM, Koutselinis AS. (2003). Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur J Surg Oncol 29:571-574. [DOI] [PubMed] [Google Scholar]
- van Miltenburg MH, van Nimwegen MJ, Tijdens I, Lalai R, Kuiper R, Klarenbeek S, Schouten PC, de Vries A, Jonkers J, van de, Water B. (2014). Mammary gland-specific ablation of focal adhesion kinase reduces the incidence of p53-mediated mammary tumour formation. Br J Cancer 110:2747-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Tanjoni I, Uryu S, Nam JO, Mielgo A, Tomar A, Luo H, Phillips A, Kwok C, Patel N, McMahon G, Stupack DG, Schlaepfer DD. (2010). Oral delivery of PND-1186 FAK inhibitor decreases spontaneous breast to lung metastasis in pre-clinical tumor models. Cancer Biology & Therapy 9:776-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Liu T, Zhu CZ, Li Y, Sun R, Sun CY, Wang AX. (2005). [Expression of KAI1, MRP-1, and FAK proteins in lung cancer detected by high-density tissue microarray]. Ai Zheng 24:1091-1095. [PubMed] [Google Scholar]
- Wang Y, Shi J, Chai K, Ying X, Zhou BP. (2013). The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets 13:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner TM, Liu ET, Craven RJ, Cance WG. (1993). Expression of focal adhesion kinase gene and invasive cancer. Lancet 342:1024-1025. [DOI] [PubMed] [Google Scholar]
- Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. (2008). Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J Cell Biol 181:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt MK, Schiemann BJ, Parvani JG, Lee YH, Kang Y, Schiemann WP. (2013). TGF-beta stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer. Oncogene 32:2005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gan B, Yoo Y, Guan JL. (2005). FAK-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev Cell 9:185-196. [DOI] [PubMed] [Google Scholar]
- Xu LH, Owens LV, Sturge GC, Yang X, Liu ET, Craven RJ, Cance WG. (1996). Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Diff 7:413-418. [PubMed] [Google Scholar]
- Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Jr., Craven RJ, Cance WG. (2000). The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem 275:30597-30604. [DOI] [PubMed] [Google Scholar]
- Xu LH, Yang X, Craven RJ, Cance WG. (1998). The COOH-terminal domain of the focal adhesion kinase induces loss of adhesion and cell death in human tumor cells. Cell Growth Differ 9:999-1005. [PubMed] [Google Scholar]
- Yom CK, Noh DY, Kim WH, Kim HS. (2011). Clinical significance of high focal adhesion kinase gene copy number and overexpression in invasive breast cancer. Breast Cancer Res Treat 128:647-655. [DOI] [PubMed] [Google Scholar]
- Zachary I. (1997). Focal adhesion kinase. Int J Biochem Cell Biol 29:929-934. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL. (2009). Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 28:35-49. [DOI] [PubMed] [Google Scholar]
- Zhao X, Peng X, Sun S, Park AY, Guan JL. (2010). Role of kinase-independent and -dependent functions of FAK in endothelial cell survival and barrier function during embryonic development. J Cell Biol 189:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhonghua S, Al-Naami A, Liaqat AK. (2012). Perforated duodenal ulcer associated with anterior abdominal abscess: A case report. Australas Med J 5:14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]