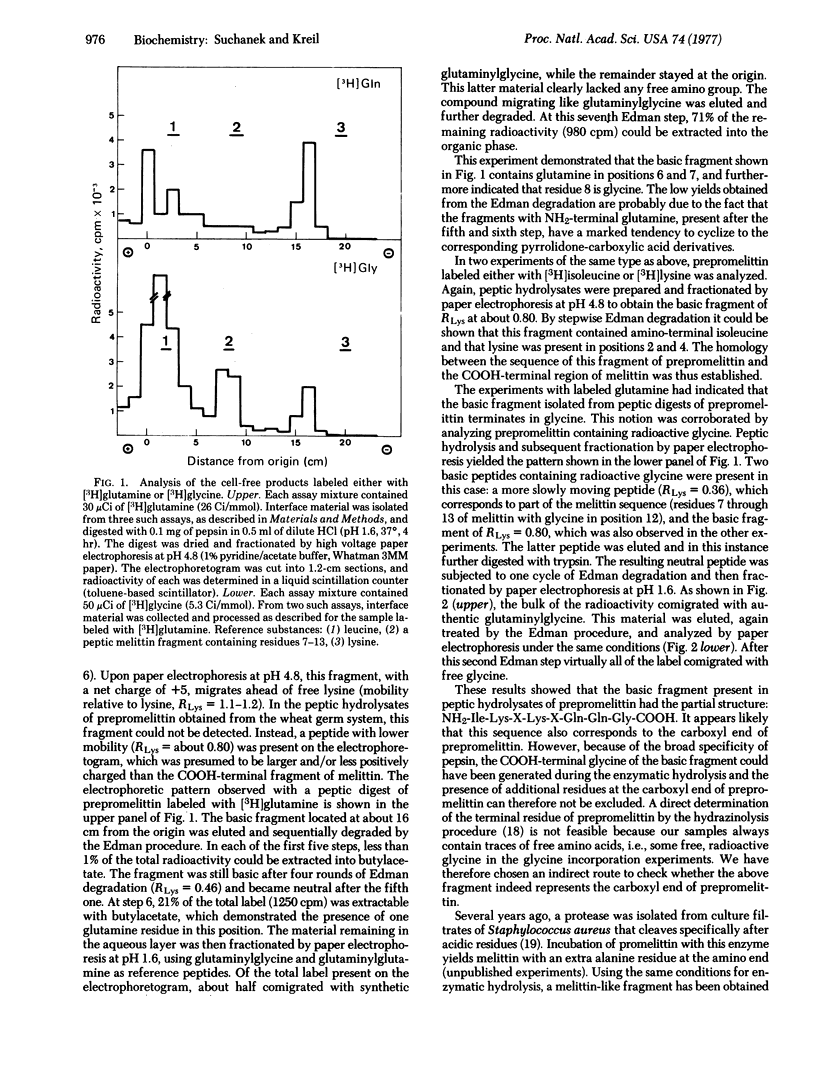
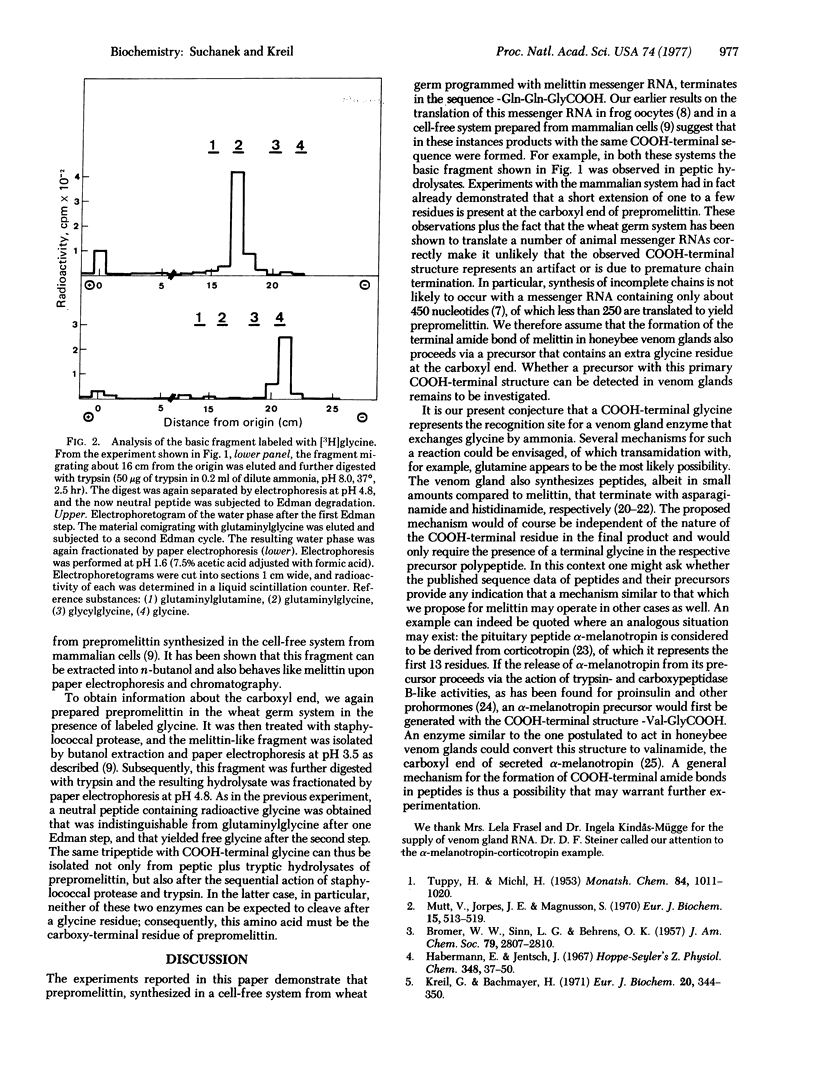
Abstract
Melittin messenger RNA from queen bee venom glands has been translated in a cell-free system from wheat germ. A product larger than promelittin is formed which has the carboxy-terminal sequence-Gln-Gln-GlyCOOH. Melittin and promelittin from venom glands terminate in -Gln-GlnCONH2. The possible role of the extra glycine residue in the formation of a COOH-terminal amide via a transamidase-like reaction is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boime I., Boguslawski S., Caine J. The translation of a human placental lactogen mRNA fraction in heterologous cell-free systems: the synthesis of a possible precursor. Biochem Biophys Res Commun. 1975 Jan 6;62(1):103–109. doi: 10.1016/s0006-291x(75)80411-6. [DOI] [PubMed] [Google Scholar]
- Callewaert G. L., Shipolini R., Vernon C. A. The disulphide bridges of apamin. FEBS Lett. 1968 Aug;1(2):111–113. doi: 10.1016/0014-5793(68)80033-x. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. I., LERNER A. B. Amino-acid sequence of the alpha-melanocyte-stimulating hormone. Nature. 1957 Jun 29;179(4574):1346–1347. doi: 10.1038/1791346a0. [DOI] [PubMed] [Google Scholar]
- Habermann E., Jentsch J. Sequenzanalyse des Melittins aus den tryptischen und peptischen Spaltstücken. Hoppe Seylers Z Physiol Chem. 1967 Jan;348(1):37–50. [PubMed] [Google Scholar]
- Haux P. Die Aminosäurensequenz von MCD-Peptid, einem spezifisch Mastzellen-degranulierenden Peptid aus Bienengift. Hoppe Seylers Z Physiol Chem. 1969 May;350(5):536–546. [PubMed] [Google Scholar]
- Haux P., Sawerthal H., Habermann E. Sequenzanalyse des Bienengift-Neurotoxins (Apamin) aus seinen tryptischen und chymotryptischen Spaltstücken. Hoppe Seylers Z Physiol Chem. 1967 Jun;348(6):737–738. [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindas-Mügge I., Lane C. D., Kreil G. Insect protein synthesis in frog cells: the translation of honey bee promelittin messenger RNA in Xenopus oocytes. J Mol Biol. 1974 Aug 15;87(3):451–462. doi: 10.1016/0022-2836(74)90096-5. [DOI] [PubMed] [Google Scholar]
- Kindås-Mügge I., Frasel L., Diggelmann H. Characterization of promelittin messenger RNA from the venom gland of young queen bees. J Mol Biol. 1976 Jul 25;105(1):177–181. doi: 10.1016/0022-2836(76)90202-3. [DOI] [PubMed] [Google Scholar]
- Kreil G., Bachmayer H. Biosynthesis of melittin, a toxic peptide from bee venom. Detection of a possible precursor. Eur J Biochem. 1971 Jun 11;20(3):344–350. doi: 10.1111/j.1432-1033.1971.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Kreil G. Biosynthesis of melittin, a toxic peptide from bee venom. Amino-acid sequence of the precursor. Eur J Biochem. 1973 Mar 15;33(3):558–566. doi: 10.1111/j.1432-1033.1973.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E., Magnusson S. Structure of porcine secretin. The amino acid sequence. Eur J Biochem. 1970 Sep;15(3):513–519. doi: 10.1111/j.1432-1033.1970.tb01034.x. [DOI] [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Ratcliffe J. G., Rees L. H., Landon J., Bennett H. P., Lowry P. J., McMartin C. Pituitary peptide. Nat New Biol. 1973 Jul 18;244(133):65–67. doi: 10.1038/newbio244065a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Suchanek G., Kindås-Mügge I., Kreil G. Translation of honeybee promelittin messenger RNA. Formation of a larger product in a mammalian cell-free system. Eur J Biochem. 1975 Dec 1;60(1):309–315. doi: 10.1111/j.1432-1033.1975.tb21005.x. [DOI] [PubMed] [Google Scholar]
- Sussman P. M., Tushinski R. J., Bancroft F. C. Pregrowth hormone: product of the translation in vitro of messenger RNA coding for growth hormone. Proc Natl Acad Sci U S A. 1976 Jan;73(1):29–33. doi: 10.1073/pnas.73.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]



