Abstract
Aims
The association of QRS duration (QRSd) with morbidity and mortality is understudied in patients with atrial fibrillation (AF). We sought to assess any association of prolonged QRS with increased risk of death or hospitalization among patients with AF.
Methods and results
QRS duration was retrieved from the baseline electrocardiograms of patients enroled in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study and divided into three categories: <90, 90–119, ≥120 ms. Cox models were applied relating the hazards of mortality and hospitalizations to QRSd. Among 3804 patients with AF, 593 died and 2305 were hospitalized. Compared with those with QRS < 90 ms, patients with QRS ≥ 120 ms, had an increased mortality [hazard ratio (HR) 1.61, 95% confidence interval (CI): 1.29–2.03, P < 0.001] and hospitalizations (HR 1.14, 95% CI: 1.07–1.34, P = 0.043) over an average follow-up of 3.5 years. Importantly, for patients with QRS 90–119 ms, mortality and hospitalization were also increased (HR 1.31, P = 0.005 and 1.11, P = 0.026, respectively). In subgroup analysis based on heart failure (HF) status (previously documented or ejection fraction <40%), mortality was increased for QRS ≥ 120 ms patients with (HR 1.87, P < 0.001) and without HF (HR 1.63, P = 0.02). In the QRS 90–119 ms group, mortality was increased (HR 1.38, P = 0.03) for those with HF, but not significantly among those without HF (HR 1.23, P = 0.14).
Conclusion
Among patients with AF, QRSd ≥ 120 ms was associated with a substantially increased risk for mortality (all-cause, cardiovascular, and arrhythmic) and hospitalization. Interestingly, an increased mortality was also observed among those with QRS 90–119 ms and concomitant HF.
Keywords: Atrial fibrillation, Heart failure, QRS duration, Bundle branch block, Arrhythmia
What's new?
Two new categories of QRS duration that carry an elevated risk of death and hospitalization among patients with atrial fibrillation.
A new never before reported QRS group with higher mortality among patients with atrial fibrillation.
QRS duration ≥120 ms increased the risk for mortality (all-cause, cardiovascular, and arrhythmic).
QRS duration ≥120 ms increased the risk for hospitalization.
Patients with a QRS 90–119 ms and concomitant heart failure had an increased mortality.
Introduction
Atrial fibrillation (AF) is the most common sustained heart rhythm disturbance worldwide, affecting an estimated 2.3 million adults in the USA and 4.5 million adults in the EU.1,2 The presence of AF alone is associated with a higher morbidity and mortality compared with normal sinus rhythm.2,3 As the population and life expectancy increase worldwide, the prevalence of AF will continue to increase.1
With ageing, the myocardium naturally degenerates, accelerated by various stressors (e.g. ischaemia, hypertension, fluid overload), leading to apoptosis and fibrosis.4 This process affects the myocytes and the cardiac conduction system (from the sinus node to the His–Purkinje system),5 often leading to an increase in the QRS duration (QRSd) over time. Thus, the prevalence of QRS prolongation increases from <1% in the first few decades of life to >10% after the eighth decade.6
The prognostic value of a wide QRS >120 ms among patients in sinus rhythm is well established. Aside from young patients with no underlying heart disease,7–10 a prolonged QRS clearly predicts increased morbidity and mortality for older patients and those with cardiovascular disease.11–13 The association of QRS prolongation and mortality is particularly well demonstrated in patients with systolic heart failure (HF) and a left bundle block branch (LBBB) of >120 ms. In this population, cardiac resynchronization therapy (CRT), will often shorten the QRS width,14 improve symptoms, increase exercise duration, and significantly decrease mortality.15,16
Although the effect of QRS prolongation in patients with sinus rhythm with or without HF has been established,17 the effect of QRS prolongation on morbidity or mortality in patients with AF, especially among those with lesser degrees of QRS prolongation (90–119 ms) and those without HF, is not fully understood. Atrial fibrillation is often present in populations with cardiovascular comorbidities, but also contributes to the morbidity and mortality of these patients. The possibility of further incremental risk based on QRS prolongation in AF has not been explored. Therefore, this study sought to determine whether patients with AF enroled in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) trial were at increased risk for death and hospitalization based on various degrees of QRS prolongation and the presence or the absence of HF.
Methods
Study cohort and data acquisition
The AFFIRM trial design, baseline characteristics, and results have been published previously.18 In brief, the study enroled 4060 patients that had AF and at least one other condition associated with a high risk for stroke and death (age ≥65, congestive HF, hypertension, diabetes, poor left ventricular function, a large left atrium, or prior stroke or transient ischaemic attack). These patients were randomized to rate control vs. rhythm control over a 4-year period with a mean follow-up of 3.5 years. All patients provided informed consent to participate in the AFFIRM study, and all participating institutions received approval from their respective institutional review boards. Patients were seen for follow-up at 2 and 4 months after randomization and then every 4 months up to a maximum of 6 years. After approval from the University of Kentucky Institutional Review Board, a formal request was submitted through the Biological Specimen and Data Repository Information Coordinating Center to obtain archived data on the patients enroled in the AFFIRM study.
The QRSd was obtained from the electrocardiogram (EKG) recorded at the time of enrolment in the AFFIRM study (longest QRS interval in the precordial leads measured by the study investigator at each participating site). The QRSd categories18 were divided into three categories: <90 ms as the reference category; 90–119 and ≥120 ms as the two comparator categories. These categories allow the selection of three groups of patients with clearly delineated QRS width: narrow (<90 ms), wide (>120 ms), and intermediate (90–119 ms). Importantly, the EKGs were not available for additional EKG review, which also precluded more detailed analyses of QRS-specific patterns. Patients with pacemakers or defibrillators at the time of randomization were excluded from analysis to prevent the potential confounding effect of prolonged QRSd due to pacing.
Cardiovascular mortality was defined as death due to stroke, pulmonary emboli, aortic events, arrhythmias, HF, or cardiac surgery/interventions. Other deaths were considered non-cardiovascular. The cause of death and non-fatal endpoints were adjudicated by a committee blinded to the therapy received.
Statistical methods
Univariate and multivariate Cox proportional hazards models were employed throughout the entire cohort to assess the impact of QRSd on the clinical outcomes of mortality (all-cause, cardiovascular, and arrhythmic) as well as hospitalization (all-cause and cardiac).
Mortality and hospitalization outcomes were also examined in multivariate analysis within two subgroups: patients with HF, defined by history of congestive HF and/or documented ejection fraction (EF) <40%; and patients without HF, defined by no history of congestive HF and EF ≥40%. Such HF definition allowed comparison of patients with normal EF and no systolic/diastolic HF to patients with low EF and/or abnormal HF status. Patients without a clear HF status as defined above (e.g. no recorded EF at baseline) were included in the overall cohort analyses but were excluded from the HF subgroups analyses.
The multivariate Cox models controlled for the AFFIRM treatment arms (rhythm control vs. rate control), baseline comorbidities (coronary artery disease; hepatic or renal disease; diabetes; cardiomyopathy; valvular disease; stroke; peripheral vascular disease; pulmonary disease), time-dependent covariates (amiodarone use; digoxin use), and propensity scores. The propensity scores were obtained from proportional odds models with the QRS category as the dependent variable and the following independent variables: gender, age >75, myocardial infarction, coronary artery bypass grafting, interventional procedure, congenital heart disease, bradycardia/atrioventricular (AV) block, angina, first episode of AF, AF accompanied by ≥1 symptoms, ventricular heart rate ≥100 b.p.m., hospitalization for qualifying AF episode, cardioversion since the onset of qualifying AF episode, antiarrhythmic (AAD) drug failure, beta-blockers, lipid-lowering agent, angiotensin/angiotensin-converting enzyme inhibitor, oestrogen replacement, and any of these drugs started/continued as initial therapy: amiodarone, sotalol, diltiazem, verapamil, beta–blockers, and class I drugs (disopyramide, flecanide, moricizine, procainamide, propafenone, or quinidine). When not used as a stratification variable (in subgroup analyses), HF was included in the multivariate Cox models as a predictor variable with three categories: HF present, as defined above by consideration of patient history and EF; HF not present; and HF status not specified.
Two forms of sensitivity analysis were also performed:
First, QRS was treated as a continuous variable instead of as a categorical variable in the multivariate Cox models for the mortality outcomes in the full cohort, to quantify a ‘width–response’ relationship between QRS and the mortality outcomes. Here, the role of propensity scores was played by fitted QRS values that were generated from linear regression models with QRS as the dependent variable and independent variables the same as in the aforementioned proportional odds models.
Secondly, the multivariate Cox models in the full cohort were re-fit to allow for the possibility that the hazard ratio (HR) comparing patients with QRS ≥ 120 ms to those with QRS < 90 ms (and, similarly, the HR comparing patients with QRS 90–119 ms to those with QRS < 90 ms) might differ for patients in sinus rhythm vs. for patients in AF at baseline; this possibility is referred to as ‘interaction’, and we tested a null hypothesis of no interaction for the three mortality and two hospitalization outcomes in the full cohort.
A P < 0.05 was considered statistically significant. Kaplan–Meier curves stratifying patients by QRSd and/or HF were also constructed for selected outcomes. Version: 9.3 of SAS software was used to fit the Cox models and obtain the Kaplan–Meier curves.
Results
Overall cohort
Among the 4060 AFFIRM patients, 256 were excluded from this analysis (250 with a pacemaker/defibrillator and 6 with no available QRS width at baseline). Among the 3804 remaining patients, 2005 (52.7%) had a QRSd of <90 ms; 1307 (34.4%) had a QRSd 90–119 ms, and 492 (12.9%) had a QRSd ≥ 120 ms. In this cohort, 593 (15.6%) died (303 from cardiovascular causes, including 148 arrhythmic death) and 2305 were hospitalized (1529 for cardiac causes) during follow-up (mean 3.5 years). At the time of randomization, 1995 (52.4%) patients were in sinus rhythm, 1660 (43.6%) were in AF, and 149 (3.9%) had an unspecified rhythm. During the study, 548 patients (14.4%) were in sinus rhythm and 603 patients (15.8%) were in AF during their initial baseline and all follow-up EKGs. The remaining patients had EKGs alternating between sinus rhythm and AF.
Univariate analysis
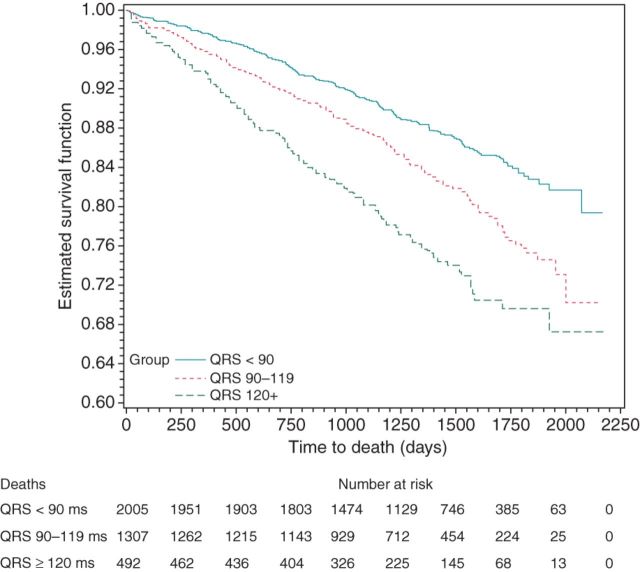
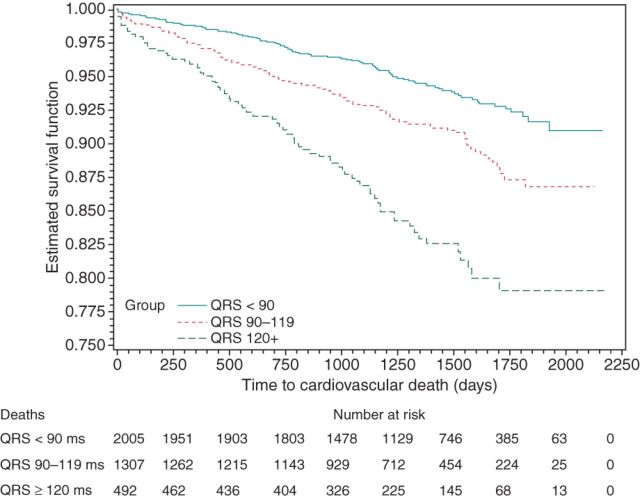
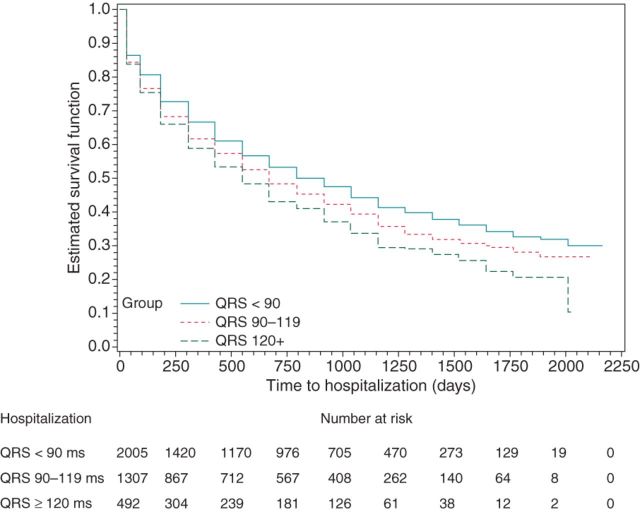
A QRS 90–119 ms (compared with QRS < 90 ms) was associated with significantly increased risk for total mortality [estimated HR 1.47, 95% confidence interval (CI) 1.23–1.76, P < 0.0001; cf. Figure 1], cardiovascular mortality (HR 1.65, 95% CI: 1.27–2.14, P = 0.0002; cf. Figure 2), arrhythmic mortality (HR 1.91, 95% CI: 1.30–2.79, P = 0.001), and all-cause hospitalization (HR 1.15, 95% CI: 1.05–1.26, P = 0.002; cf. Figure 3).
Figure 1.
Kaplan–Meier curves for death by QRS width.
Figure 2.
Kaplan–Meier curves for cardiovascular death by QRS width.
Figure 3.
Kaplan–Meier curves for hospitalization by QRS width.
Moreover, a QRS ≥ 120 ms (compared with QRS < 90 ms) was associated with significantly increased risk for total mortality (HR 2.20, 95% CI: 1.76–2.73, P < 0.0001; cf. Figure 1), cardiovascular mortality (HR 3.10, 95% CI: 2.31–4.14, P < 0.0001; cf. Figure 2), arrhythmic mortality (HR 3.93, 95% CI: 2.60–5.95, P < 0.0001), all-cause hospitalization (HR 1.31, 95% CI: 1.16–1.49, P < 0.0001; cf. Figure 3), and cardiac hospitalization (HR 1.41, 95% CI: 1.21–1.63, P < 0.0001).
Multivariate analysis
After controlling for other independent variables using multivariate Cox models, a QRSd ≥ 120 ms was associated with significantly increased risk for the following outcomes: all-cause death (Figure 4A), cardiovascular death (Figure 4B), and arrhythmic death (Figure 4C), plus all-cause and cardiac hospitalizations (HR 1.14, 95% CI: 1.00–1.30, P = 0.043 and HR 1.17, 95% CI: 1.01–1.37, P = 0.038, respectively). Patients with a QRSd between 90 and 119 ms also had a significantly increased risk of all-cause (Figure 4A), cardiovascular (Figure 4B), and arrhythmic death (Figure 4C) as well as all-cause hospitalization (HR 1.11, 95% CI: 1.01–1.22, P = 0.026).
Figure 4.
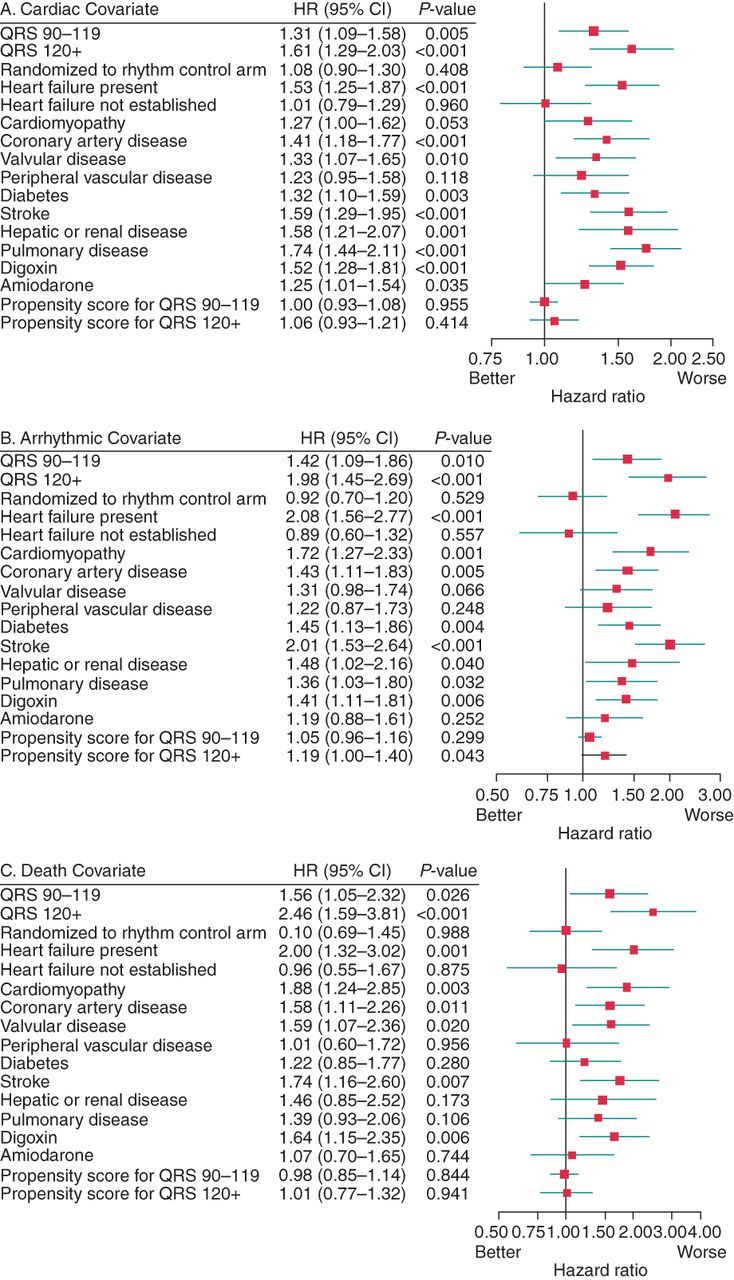
(A, B, and C): Results from Cox model for all-cause (A), cardiac (B), arrhythmic (C) death. The significant predictors for all-cause, cardiac, and arrhythmic mortality are those whose P values are <0.05 and includes classic, well-known predictors such as coronary artery disease, stroke, or system organ failure (renal, hepatic, or pulmonary). Of major interest in these figures are the statistically significant hazard ratios (HR) for all-cause, cardiac, and arrhythmic mortality, respectively, for QRS 90–119 ms (Row 1 in figures A, B, and C) and for QRS > 120 ms (Row 2 in figures A, B, and C) compared with the QRS reference <90 ms.
Results based on heart failure status
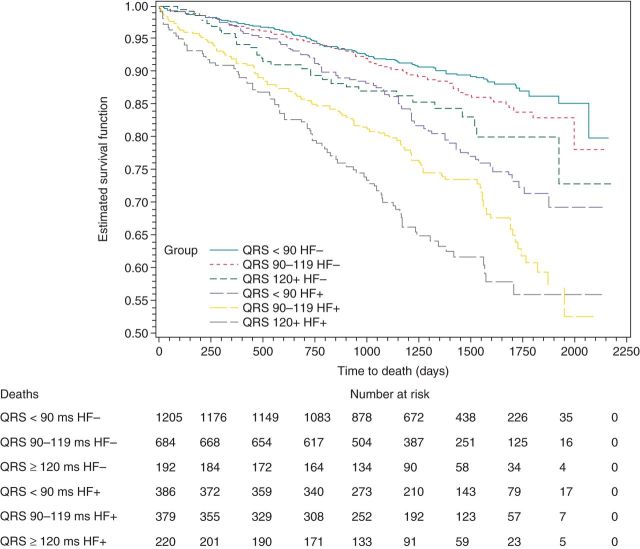
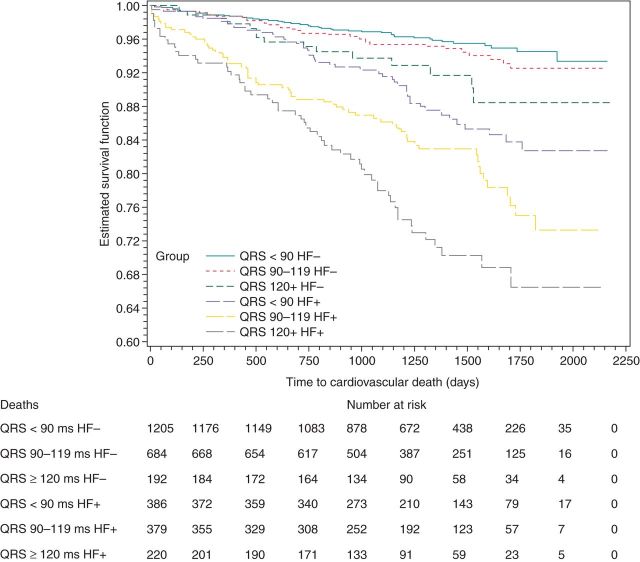
Among patients with QRSd < 90 ms, 386 (24.3%) had HF vs. 1205 (75.7%) without HF. Among patients with QRSd between 90 and 119 ms, 379 (35.7%) had HF vs. 684 (64.3%) without HF, and in patients with QRSd ≥ 120 ms, 220 (53.4%) had HF vs. 192 (46.6%) without HF. The presence or absence of HF was not established for 738 patients. Figures 5 and 6 graphically depict the associations of QRSd with all-cause and cardiovascular mortality based on HF status. Table 1 reports the adjusted associations of QRSd with five outcomes (all-cause, cardiovascular, and arrhythmic deaths; all-cause and cardiac hospitalization) based on HF status.
Figure 5.
Kaplan–Meier curves for death by QRS width and HF status.
Figure 6.
Kaplan–Meier curves for cardiovascular death by QRS width and HF status.
Table 1.
Adjusted associations of QRS width with endpoints by HF status
| QRS 90–119 |
QRS ≥ 120 |
|||
|---|---|---|---|---|
| HR (95% CI)a | P value | HR (95% CI)a | P value | |
| Heart failure absent | ||||
| Overall death | 1.23 (0.92–1.64) | 0.14 | 1.63 (1.09–2.43) | 0.02 |
| Cardiovascular death | 1.31 (0.84–2.05) | 0.23 | 1.87 (1.04–3.37) | 0.04 |
| Arrhythmic death | 1.04 (0.53–2.02) | 0.91 | 2.08 (0.92–4.70) | 0.08 |
| Overall hospitalization | 1.18 (1.05–1.34) | 0.01 | 1.24 (1.01–1.51) | 0.04 |
| Cardiac hospitalization | 1.08 (0.92–1.27) | 0.34 | 1.37 (1.09–1.74) | <0.01 |
| Heart failure present | ||||
| Overall death | 1.38 (1.03–1.86) | 0.03 | 1.87 (1.35–2.58) | <0.001 |
| Cardiovascular death | 1.48 (1.01–2.18) | 0.04 | 2.17 (1.45–3.26) | <0.001 |
| Arrhythmic death | 2.41 (1.32–4.39) | <0.01 | 3.49 (1.87–5.54) | <0.0001 |
| Overall hospitalization | 1.01 (0.84–1.20) | 0.93 | 1.12 (0.91–1.38) | 0.28 |
| Cardiac hospitalization | 1.03 (0.84–1.28) | 0.75 | 1.18 (0.92–1.50) | 0.19 |
aHR, estimated hazard ratio.
CI, confidence interval.
Comparisons are with patients with QRS < 90; covariates are listed in the Statistical Methods Section.
Among those without HF, QRSd ≥ 120 ms was associated with a significantly increased risk of all outcomes except arrhythmic death; although not significant (P = 0.08), a trend was observed with the estimated hazard of arrhythmic death more than doubled. Among those with HF, a QRSd ≥ 90 ms was associated with a significant increased risk of death (all-cause, cardiovascular, and arrhythmic death), with greatest risks for those with a QRS ≥ 120 ms.
Additional analyses
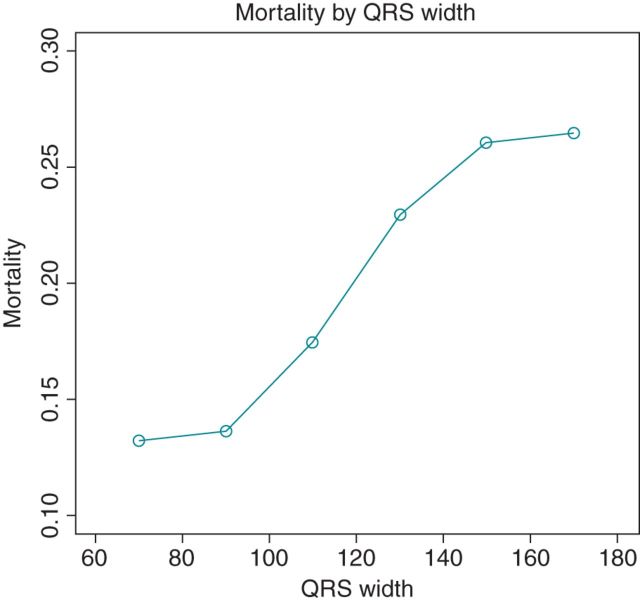
QRS used as a continuous variable
Figure 7 displays the unadjusted proportions of patients who died during follow-up, based on 20 ms increments of QRSd, suggesting that mortality increases progressively along with the greater QRSd. This suggestion is substantiated by findings from the multivariate Cox models, in which QRS was treated as a continuous variable. More specifically, each 20 ms increase in QRSd increases the hazard of mortality by 12% (HR 1.12, 95% CI: 1.04–1.21, P = 0.002). Moreover, each 20 ms increase in QRSd increases the hazard of cardiovascular mortality by 16% (HR 1.16, 95% CI: 1.05–1.28, P = 0.003) and of arrhythmic mortality by 20% (HR 1.20, 95% CI: 1.05–1.37, P = 0.009).
Figure 7.
Results from continuous analysis of QRSd for total mortality.
QRS based on EKG's rhythm at baseline
No significant interactions between the patient's heart rhythm status at baseline (sinus rhythm or AF) and the QRSd were observed in the multivariate Cox models for the five endpoints of all-cause mortality, cardiovascular mortality, arrhythmic mortality, hospitalization, and cardiac hospitalization (P values of 0.78, 0.15, 0.41, 0.19, and 0.23, respectively). Therefore, the ability of QRSd to predict death and hospitalization was not specific to a patient's heart rhythm status at baseline.
Discussion
This study demonstrates a significant association between QRSd and morbidity/mortality in a large AF cohort during the 3.5-year follow-up. In patients with HF, either mild (90–119 ms) or severe prolongation (≥120 ms) of QRSd was associated with an increased mortality. Likewise, the QRS prolongation ≥120 ms among patients with no HF was associated with increased mortality. These results identify new subgroups of patients with AF at increased risk for death and hospitalization, based on the QRSd.
QRS prolongation in patients without atrial fibrillation
Asymptomatic QRS prolongation
The relation between QRSd and morbidity/mortality varies depending on age. Young, asymptomatic patients without cardiovascular disease have normal life expectancy despite QRS prolongation, in the form of a LBBB or right bundle block branch (RBBB).7 In contrast, QRS prolongation among older asymptomatic patients portends worse outcomes as demonstrated in the Swedish Primary Prevention Study.19 Among 7392 middle-aged men, subjects with LBBB were more likely to progress to complete heart block (HR 12.9, 95% CI: 4.1–40.2) and had greater all-cause mortality (HR 1.85, 95% CI: 1.15–2.97) over the 28-year follow-up. Similarly, subjects with RBBB were more likely to progress (>four-fold increase risk) to a high-degree AV block or need a pacemaker implantation compared with those without a BBB (HR = 3.64, 95% CI: 0.79–16.7). Similar results were found with a 2.5- to 10-fold increase in risk for sudden cardiac death among patients with prolonged QRS in two large observational studies.20,21
Cardiovascular disease and QRS prolongation
In large cohorts with cardiovascular disease, QRSd ≥ 120 ms is related to adverse events in the presence of concomitant coronary artery disease or other conduction abnormalities such as heart block.7,8 Worse outcomes are also observed among patients with milder QRS prolongation (>106 ms)11 and hypertension and specific populations with cardiovascular disease: coronary artery disease, diabetes, acute myocardial infarction, hypertrophic cardiomyopathy, and aortic stenosis.12,22–25 The worst clinical outcomes are undoubtedly in patients with systolic HF, EF < 35%, and LBBB.26–28 These patients have improved morbidity and mortality14,29 when the QRS width is corrected with CRT,16 but most previous studies had relatively few or no patients with AF.29,30
QRS prolongation in patients with atrial fibrillation
Even though AF and complete BBB are individually recognized as important predictors of morbidity and mortality,3,31,32 the relationship between QRSd and morbidity/mortality in patients with AF is poorly studied, especially among those with mild degrees of QRS prolongation (90–119 ms) or those without HF. Among the 669 patients from the In-CHF database, the presence of a complete LBBB and AF was associated with increased 1-year mortality (HR 1.88; 95% CI: 1.37–2.57) and 1-year hospitalization (HR 1.83; 95% CI: 1.26–2.67).33 The large cohort and long-term follow-up of the AFFIRM trial overcome previous limitations of smaller studies with shorter follow-up, while demonstrating similar increases in morbidity and mortality with QRS prolongation among patients with AF. Such findings, already extensively demonstrated in sinus rhythm, may have clinical implications. It reinforces for instance the theoretical ground for the benefit of biventricular pacing in AF patients, for whom the benefits of cardiac resynchronization have been much less studied than for those in sinus rhythm.
Furthermore, two novel findings with potential clinical implications arise from our analysis.
First, HF patients with AF can be further risk stratified based on the extent of QRS prolongation. In addition to patients with a QRS ≥ 120 ms having the highest risks for all-cause, cardiovascular, and arrhythmic death, patients with a lesser degree of QRS prolongation (between 90 and 119 ms) also have significantly elevated risks of these adverse outcomes (compared with QRS < 90 ms). There was even a ‘width–response relationship’ between QRS prolongation and mortality when the QRSd was treated as a continuous variable, with progressively higher mortality corresponding to further QRS prolongation. Such a progressive increase in mortality based on the extent of QRS prolongation has not been previously demonstrated in a large cohort of patients with AF. The association of QRS prolongation with mortality may reflect advanced myocardial disease, known to progressively alter the myocardial structure including the His–Purkinje conduction system. On the other hand, QRS prolongation from normal duration (<90 ms) to an incomplete BBB (90–119 ms) may result in cardiac dyssynchrony.34,35 In a ventricle already stressed from impaired systolic function and loss of atrial contraction, this may contribute to worsening HF by increasing myocardial demand. Incomplete BBB may worsen left ventricular preload and reduce left ventricular filling, especially when AF is associated with a rapid ventricular response. However, attempts to resynchronize ventricular depolarization with biventricular pacemakers in patients with incomplete BBB and sinus rhythm have not improved clinical outcomes.36 Regardless, a moderately prolonged QRSd (90–119 ms) in patients with HF and AF are at greater risk for death and hospitalization.
Secondly, our analysis suggests that a prolonged QRSd ≥ 120 ms in non-HF patients with AF is associated with worse outcomes (including all-cause and cardiovascular death, as well as total and cardiovascular hospitalizations) compared with those with normal QRSd. These results correlate with findings from previous studies among patients without AF as described above.19–21 Our analysis further extends such findings to the type of patients with AF enroled in AFFIRM.
Two hypotheses may account for decreased survival among non-HF patients with AF and QRS ≥ 120 ms. A potential explanation is that the disease in the Purkinje system manifested on EKG by QRS prolongation is a bystander marker of advanced cardiovascular disease, which leads naturally to an increased mortality. However, this hypothesis is unlikely since the increased mortality remains even after correcting for all the available important comorbidities and diseases, including the use of AAD. Therefore, the QRS prolongation itself is more likely directly responsible for worse outcomes, via the development of heart block, HF, or deleterious arrhythmias. This alternative hypothesis is reinforced by studies demonstrating progression to higher degree AV block19 and increased risk of SCD in patients with LBBB as outlined above.20,21 Additional supporting evidence originates from a cardiac magnetic resonance imaging study, where even asymptomatic patients without underlying cardiovascular disease but with a complete LBBB have decreased left ventricular systolic function compared with patients without BBB (54 vs. 62% P < 0.005).37 It remains to be determined whether earlier and/or additional therapies (such as right ventricular or biventricular pacemaker) may improve the outcomes of those patients.
Study limitations
A clear limitation is the non-accessibility of the study EKGs for additional review that precludes us from describing specific QRS patterns (for instance left vs. right BBB). However, one may place confidence in the results of this analysis. The high standards for the design and data collection of the National Institute of Health trials such as AFFIRM are indeed considered to be a reference for reliability. The accuracy of this analysis is further reinforced by the similar results reported previously in BBB cohorts with non-AF patients (19–24).
In AFFIRM, the QRS width was determined by the measurement of the longest interval in the precordial leads by a study investigator during enrolment, different from the current standard of measuring the QRSd, ‘from the earliest onset to the latest offset of the waveform in all lead, generally taken from a spatial vector magnitude or superimposed complexes’ (2009).38 In addition, the use of Core labs at the time of this trial was not of standard practice. However, the lack of current standards in EKG measurement or centralized reading should not substantially modify our results, since all EKG measurements in AFFIRM were standardized.
Potential known confounders such as pacemakers do not appear to compromise the validity of this analysis, because patients with pacemakers at baseline were excluded. Follow-up EKGs were not available at all subsequent visits. Whether patients had substantial changes to their QRS intervals during the study is unknown.
The absence of significant associations between QRS prolongation and hospitalization (both all-cause and cardiac) among HF patients with AF may relate to the sample size for this subgroup, as significant associations have been demonstrated in other studies with larger sample size.
Although the AFFIRM cohort was representative of the majority of patients with AF, these results may not apply to younger AF patients without risk factors.
Finally, the limitations inherent to any post hoc analysis are present, particularly the possibility that confounding variables may not have been recorded in the database and thus not controlled for in the analysis.
Conclusion
In patients with AF, a QRSd ≥ 90 ms is associated with significantly increased risks for death (all-cause, cardiovascular, and arrhythmic) and hospitalization (all-cause and cardiac). This risk was elevated further when the QRSd was ≥120 ms. In subgroup analysis, two types of patients had significant increases in all-cause and cardiovascular deaths: those without HF and QRS ≥ 120 ms, and those with HF and QRS ≥ 90 ms. These results identify new subgroups of patients with AF at increased risk for death and hospitalization, based on the QRSd.
Conflict of interest: none declared.
References
- 1.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–6. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, et al. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–59. doi: 10.1016/j.exger.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation. 1992;85:2176–84. doi: 10.1161/01.cir.85.6.2176. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation. 1998;98:2494. doi: 10.1161/01.cir.98.22.2494. [DOI] [PubMed] [Google Scholar]
- 7.Rotman M, Triebwasser JH. A clinical and follow-up study of right and left bundle branch block. Circulation. 1975;51:477. doi: 10.1161/01.cir.51.3.477. [DOI] [PubMed] [Google Scholar]
- 8.Fahy GJ, Pinski SL, Miller DP, McCabe N, Pye C, Walsh MJ, et al. Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185. doi: 10.1016/s0002-9149(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi M, Nakano H, Kuwahara K, Masuda I, Okawa Y, Miyazaki H, et al. Prognostic and clinical significance of newly acquired complete right bundle branch block in Japan Airline pilots. Intern Med. 2003;42:21. doi: 10.2169/internalmedicine.42.21. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Noseworthy PA, McCarty D, Yared K, Weiner R, Wang F, et al. Significance of electrocardiographic right bundle branch block in trained athletes. Am J Cardiol. 2011;107:1083. doi: 10.1016/j.amjcard.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 11.Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Jern S, Dahlöf B, et al. QRS Duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: the losartan intervention for endpoint reduction in hypertension study. Hypertension. 2004;43:1029–34. doi: 10.1161/01.HYP.0000125230.46080.c6. [DOI] [PubMed] [Google Scholar]
- 12.Greve AM, Gerdts E, Boman K, Gohlke-Baerwolf C, Rossebø AB, Devereux RB, et al. Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis. The SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study. J Am Coll Cardiol. 2012;59:1142–9. doi: 10.1016/j.jacc.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:14. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 14.Rickard J, Popovic Z, Verhaert D, Sraow D, Baranowski B, Martin DO, et al. The QRS narrowing index predicts reverse left ventricular remodeling following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34:604–11. doi: 10.1111/j.1540-8159.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 15.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 16.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Comparison of medical therapy, pacing, and defibrillation in heart failure (COMPANION) investigators. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 17.El-Chami MF, Brancato C, Langberg J, Delurgio DB, Bush H, Brosius L, et al. QRS duration is associated with atrial fibrillation in patients with left ventricular dysfunction. Clin Cardiol. 2010;33:132–8. doi: 10.1002/clc.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. Atrial fibrillation follow-up investigation of rhythm management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson P, Wilhelmsen L, Rosengren A. Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Göteborg, Sweden. Eur Heart J. 2005;26:2300. doi: 10.1093/eurheartj/ehi580. [DOI] [PubMed] [Google Scholar]
- 20.Rabkin SW, Mathewson FA, Tate RB. Natural history of left bundle-branch block. Br Heart J. 1980;43:164. doi: 10.1136/hrt.43.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurl S, Mäkikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–94. doi: 10.1161/CIRCULATIONAHA.111.025577. [DOI] [PubMed] [Google Scholar]
- 22.Wong CK, Stewart RAH, Gao W, French JK, Raffel C, White HD. Prognostic differences between different types of bundle branch block during the early phase of acute myocardial infarction: insights from the Hirulog and Early Reperfusion or Occlusion (HERO)-2 Trial. Eur Heart J. 2006;27:21. doi: 10.1093/eurheartj/ehi622. [DOI] [PubMed] [Google Scholar]
- 23.Brilakis ES, Wright RS, Kopecky SL, Reeder GS, Williams BA, Miller WL. Bundle branch block as a predictor of long-term survival after acute myocardial infarction. Am J Cardiol. 2001;88:205. doi: 10.1016/s0002-9149(01)01626-5. [DOI] [PubMed] [Google Scholar]
- 24.Sumner G, Salehian O, Yi Q, Healey J, Mathew J, Al-Merri K, et al. HOPE Investigators. The prognostic significance of bundle branch block in high-risk chronic stable vascular disease patients: a report from the HOPE trial. J Cardiovasc Electrophysiol. 2009;20:781. doi: 10.1111/j.1540-8167.2009.01440.x. [DOI] [PubMed] [Google Scholar]
- 25.Bongioanni S, Bianchi F, Migliardi A, Gnavi R, Pron PG, Casetta M, et al. Relation of QRS duration to mortality in a community-based cohort with hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:503–6. doi: 10.1016/j.amjcard.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–92. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 27.Buliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. Department of veterans affairs survival trial of antiarrhythmic therapy in congestive heart failure. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–91. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 28.Gottipaty VK, Krelis SP, Lu F, Spencer EP, Shusterman V, Weiss R, et al. The VEST investigators. The resting electrocardiogram provides a sensitive and inexpensive marker of prognosis in patients with chronic congestive heart failure. J Am Coll Cardiol. 1999;33:145A. [Google Scholar]
- 29.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 30.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 31.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol. 1998;32:695. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 32.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 33.Baldasseroni S, De Biase L, Fresco C, Marchionni N, Marini M, Masotti G, et al. Italian Network on Congestive Heart Failure. Cumulative effect of complete left bundle-branch block and chronic atrial fibrillation on 1-year mortality and hospitalization in patients with congestive heart failure. A report from the Italian network on congestive heart failure (in-CHF database) Eur Heart J. 2002;23:1692–8. doi: 10.1053/euhj.2001.3157. [DOI] [PubMed] [Google Scholar]
- 34.Fauchier L, Marie O, Casset-Senon D, Babuty DD, Cosnay PP, Fauchier JP. Reliability of QRS duration and morphology on surface electrocardiogram to identify ventricular dyssynchrony in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:341–4. doi: 10.1016/s0002-9149(03)00644-1. [DOI] [PubMed] [Google Scholar]
- 35.Turner MS, Bleasdale RA, Vinereanu D, Mumford CE, Paul V, Fraser AG, et al. Electrical and mechanical components of dyssynchrony in heart failure patients with normal QRS duration and left bundle-branch block. Impact of left and biventriclar pacing. Circulation. 2004;109:2544–9. doi: 10.1161/01.CIR.0000131184.40893.40. [DOI] [PubMed] [Google Scholar]
- 36.Beshai JF, Grimm RA, Nagueh SF, Baker JH, II, Beau SL, Greenberg SM, et al. Rethin Q study investigators. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–71. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 37.Grines Cl, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF. Functional abnormalities in isolated left bundle branch block. The efforts of interventricular asynchrony. Circulation. 1989;79:845–53. doi: 10.1161/01.cir.79.4.845. [DOI] [PubMed] [Google Scholar]
- 38.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, et al. American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; HEART Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:976–81. doi: 10.1016/j.jacc.2008.12.013. [DOI] [PubMed] [Google Scholar]