Abstract
[Purpose] The aim of this study was to determine whether neck muscle fatigue affects dynamic visual acuity in healthy young participants. [Subjects and Methods] This study was a double-blinded, prospective, randomized, controlled trial. Thirty healthy young subjects (ages 21 to 30 years) participated in the study. Participants were randomly divided into an experimental group (n=15) and a control group (n=15). The experimental group performed an exercise designed to induce neck muscle fatigue and the control group preformed non-fatiguing sham exercises. [Results] There were significant differences in mean dynamic visual acuity between the two groups (0.26±0.11 LogMar versus 0.003±0.02 LogMar). Subjects in the experimental group showed a significant decline in their dynamic visual acuity compared with the control group. Dynamic visual acuity strongly correlated with neck muscle fatigue (r = 0.79). No significant differences in joint position error were observed between the two groups and no significant correlations between joint position error and neck muscle fatigue were observed (r = 0.23). [Conclusion] The results of this study suggest that neck muscle fatigue negatively impacts dynamic visual acuity. Although not statistically significant, cervical spine proprioception as measured by the joint position error in the experimental group was diminished after fatigue.
Key words: Dizziness, Cervical vertigo
INTRODUCTION
Postural and visual stability are dependent upon efficient and accurate central processing of visual, vestibular, and somatosensory afferent input1). This afferent input undergoes multimodal sensory integration in several areas of the brain and brainstem in order to provide efferent output to maintain postural equilibrium and oculomotor control2). Inaccurate sensory information from dysfunctional sensory end organs leads to a sensory mismatch, causing postural and/or visual instability1). Sensory mismatch can result from several causes including, but not limited to, vestibular disorders, neurological disease, pharmacology, and cervical spine trauma1, 2). Somatosensory information from the cervical spine can be altered as a result of direct trauma1, 3). Cervical spine trauma can lead to cervical spine muscle fatigue and modify the discharge firing rate of upper cervical sensory receptors1, 4, 5). Somatosensory information from the upper cervical spine is transmitted through nerve cells originating mainly from the C2 dorsal root ganglion1, 2). Mechanoreceptors in the upper cervical spine converge in the central cervical nucleus (CCN)1, 3). The CCN serves as a pathway to the cerebellum, which integrates and organizes vestibular, ocular, and proprioceptive information1, 2, 4). Disturbances in the neural connections between the three sensory systems can lead to mismatched sensory input, causing conflicts among all inputs from the different sensory systems which cause dizziness, unsteadiness, and visual disturbance1, 4). For example, altered somatosensory input, particularly from the upper cervical spine structures, can disturb the vestibular system1, 3). Clinical research has shown that when experiencing such disturbances, patients become less able to utilize vestibular information to resolve inaccurate and irregular information from the somatosensory and visual systems1, 2). Moreover, evidence suggests that upper cervical muscle fatigue may be an important contributing factor to altered postural stability in people with neck pain because neck muscle fatigue has been shown to modify the discharge of sensory receptors in neck muscles and affect proprioception2, 6, 7).
Pinsault and Vuillerme7) investigated the relationship between neck muscle fatigue and cervical spine proprioception using the joint position error (JPE) test. Subjects were asked to relocate their head back to center as accurately as possible after full active cervical rotation to the left and right sides7). Subjects were randomly allocated to cervical spine muscle fatigue and control groups7). Less accurate and less consistent cervical joint repositioning was observed in the fatigue group which were correlated with abnormal afferent input from the neck joint and muscle receptors7).
Revel et al.8) assessed cervicocephalic kinesthetic sensibility in patients with cervical pain. Their results demonstrated that patients with cervical pain had significantly less accurate head repositioning performance compared with their control group8). Heikkila and Astrom9) investigated the effects of neck somatosensory disturbance in patients with cervical whiplash trauma. They found that patients with chronic dysfunction after whiplash trauma were significantly less accurate than a control group in their ability to relocate their heads in space after actively rotating them away from the reference position9, 10).
Nicolas et al.11) investigated the effects of cervical muscular fatigue on postural control under multiple sensory conditions and determined that cervical muscle fatigue increased the center of foot pressure displacement in the absence of vision11). Their results indicated that there is a correlation between neck muscle fatigue and impaired postural stability11).
Stapley et al. examined whether patients with cervical spine whiplash injuries had an increase in postural body sway after contractions of their dorsal neck muscles12). Sway was measured during stance in 13 patients before and after performing 5 minutes of isometric dorsal neck muscle contractions12). They found that after performing the contractions, seven subjects had signs of fatigue via electromyography and increased sway12). This study demonstrated the link between neck muscle fatigue and impaired postural stability12). It also suggested that balance and postural control could be altered in healthy subjects by inducing fatigue in the neck muscles12). Deficits in oculomotor control have also been reported in patients with cervical trauma1).
Tjell and Rosenhall13) observed that altered smooth-pursuit eye movement occurred in subjects with neck trauma when their neck were rotated. These findings suggest that normal eye movement is partially dependent upon accurate sensory input from the cervical spine13). There is also evidence to suggest that the cervical spine influences eye movements via the vestibular system14). Stimulation of the deep cervical spine mechanoreceptors has a measurable impact on the vestibulo-ocular reflex (VOR)1, 4).
Pardoan et al.14) investigated the interaction between neck proprioception and the VOR14). They used a rotary chair to passively rotate 16 healthy subjects facing forward and with their heads passively turned 70 degrees to either side14). The VOR gain tended to be lower when the subjects were rotated with their heads turned in the direction opposite to the direction of rotation compared with when they were rotated in the same direction but with their head facing forward14). The results of this study suggest that there is a measurable interaction between neck proprioception and the VOR in subjects with normal vestibular function14). Also, abnormal neck muscle proprioceptive signals may give rise to asymmetric functioning of the VOR and contribute to postural and visual instability14).
Interaction between cervical spine proprioception and the VOR has been observed in a very limited number of studies and its impact on the human VOR is not fully understood1, 14, 15). The purpose of this investigation was to determine the effects of cervical muscle fatigue on dynamic visual acuity (DVA) and JPE.
SUBJECTS AND METHODS
This study was conducted in the Department of Physical Therapy, King Abdulaziz University, Jeddah, Saudi Arabia. Thirty adults participated in the study. Fifteen participants (8 males and 7 females) were randomly allocated to the experimental group and 15 participants (7 males and 8 females) were randomly allocated to the control group. Informed consent was acquired prior to the beginning of the study from participants. The study protocol was approved by the Institutional Review Board at King Abdulaziz University. Potential participants were recruited based on the following criteria: healthy young adults between the ages of 21 to 30 years without current cervical spine pain or history of cervical spine whiplash injury. Participants were required to pass all comprehensive screening tests to ensure normal function of the somatosensory, visual, and vestibular systems. Screening tests included all of the following: cervical stability (alar ligament test, Sharp-Purser test, and lateral shear test), cervical vascular screening using the modified vertebral artery test, vestibular system screening (Hallpike-Dix test, roll test, head thrust test, head shaking-induced nystagmus test, and condition 5 of the NeuroCom SMART Balance Master®system16), static and dynamic visual acuity and cervical spine somatosensory integrity (JPE). To be included in the study, all screening test results had to be negative. The study recruited a cohort of 30 participants and informed consent was obtained from each subject. A post power analysis with an effect size of 1.1 according to the change in DVA between the cervical spine muscle fatigue and control groups, an alpha level of 0.05 and a sample size of 30 indicated that the power was 0.89.
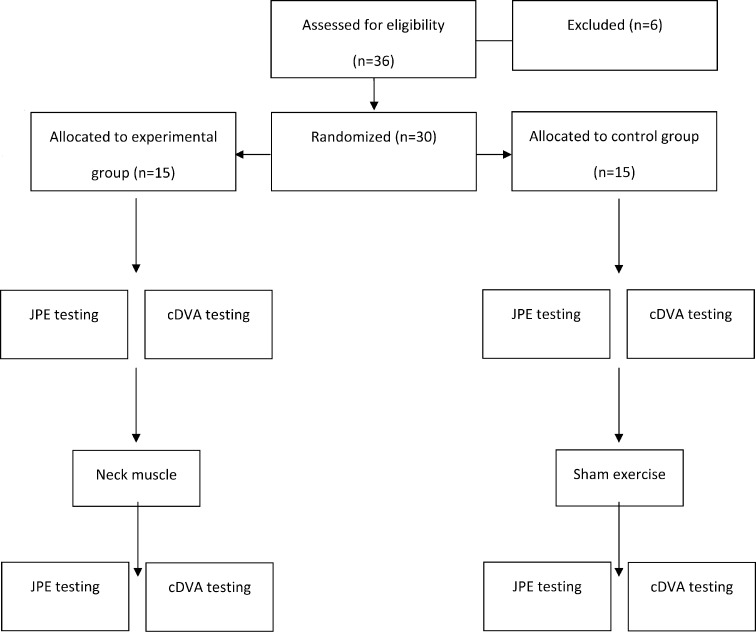
Randomization of group assignments was accomplished using a computer-generated random sequence. The two main outcome variables were the VOR as measured by the computerized dynamic visual acuity (cDVA) and cervical joint position sense as measured by the JPE test. The VOR and JPE measurements were taken before and after the intervention. Data were collected for all participants between 8 am and 12 pm to minimize the effects of normal fatigue17) (Fig. 1).
Fig. 1.
Consort flow diagram for the study
We used the computerized DVA (cDVA) InVisionTM system to determine the ability of participants accurately perceive objects while actively moving their heads. The cDVA is commonly used when evaluating patients with possible VOR dysfunction and other vestibular deficits18,19,20). Static visual acuity was determined followed by dynamic visual acuity19). The difference between the two test scores was calculated and the result was converted into LogMar, which is a measure of visual acuity loss. According to Herdman et al.19), changes of one line (0.1 LogMAR) or less (≤ 0.1) are considered normal and changes of two or more lines (> 0.2) are abnormal19). The cDVA is a reliable and sensitive tool for identifying patients with vestibular dysfunction resulting from an impaired VOR19, 20). The sensitivity of the cDVA is reported to be 94.5% and the specificity is reported to be 95.2%19). The positive predictive value is 96.3% and the negative predictive value is 93%19). We used the JPE test to measure the cervical spine somatosensory system8). Participants sat on a chair 90 cm away from a fixed target on a wall while wearing a head strap with a top-mounted laser pointer2, 22, 23). They were instructed to focus on the target then close their eyes and remember the starting position of the head, specifically the “zero target”. Keeping their eyes closed, the participants maximally rotated their heads to one side, and then returned their heads to the starting position as accurately as possible21, 23). No instructions concerning speed were given8, 22, 23). The new position of the laser light was noted and the distance from the starting point was measured (reposition error distance)9, 21, 23). The test was repeated 10 time for each rotation direction in random order8, 21). The head repositioning absolute value (AE) in centimeters was calculated8, 21). A normal JP is less than or equal to 4.5 degrees1, 8, 9, 12, 21). We used the mVAS to subjectively measure the participant’s upper posterior neck muscle fatigue level7). Participants in both groups rated their level of fatigue before and after the intervention. The mVAS was a number scale from 0 to 107, 24). A score of 0 was defined as no fatigue at all and 10 was defined as the most fatigue imaginable24). Participants in the experimental group sat on a customized neck exercise machine. With their head and neck in a neutral position, participants isometrically resisted a weight stack load for of the 5 minutes6). The load used in the weight stack was equal to 30% of the participant’s maximum voluntary contraction (MVC)6, 7) which was determined for each subject using a dynamometer. Participants performed 3 MVC and the average reading obtained from the dynamometer (in pounds) was used for represented the MVC for each participant6, 7). In order to limit trunk muscles from contributing during the contraction, a normal gait belt was used during MVC and 5 minutes isometric exercise to stabilize their trunk to the back support of the neck exercise machine6). The neck fatigue level of the participants was measured immediately following the 5 minutes of isometric exercise7).
Participants sat on a chair facing a wall while wearing a head strap with a top-mounted laser pointer attached. Four targets of the same shape and width (center, down, left, and right) were fixed to the wall at the subject’s eye level. The sham exercise began with the participant’s head in a neutral position (eyes straight ahead). Participants pointed the laser inside the large circle of the center target. From this starting position, participants were instructed to point the laser inside the second target by moving their head slightly and then returning the laser to the center target. Participants repeated the same activity for all targets as follows: center-down, center-right, and center-left for a total of 5 minutes (including two1-minute breaks). This sham exercise was designed to not fatigue the cervical muscles. The eyes of the participants were open during this exercise and no instructions concerning speed were given. Data was collected by researchers blinded to group assignment. Participants were instructed not to reveal their intervention allocation to the researchers during data collection.
Data were analyzed using the SPSS for Windows version 19.0 (SPSS, Inc., Chicago, IL, USA). Frequencies and relative frequencies were computed for gender, and means and standard deviations (SD) were calculated for the continuous variables age, DVA, and JPE. The χ2 test of independence was used to assess the relationship between gender and type of group at baseline, and the Kolmogorov-Smirnov test was applied to test for the normality of continuous variables. The Mann-Whitney U-test was performed to compare the mVAS scores between the two groups at baseline. The difference between mVAS, DVA, and JPE values at baseline and at the end of the study were calculated and these the differences were compared between the two groups using the independent sample t-test. Spearman’s correlation and regression analysis were used to examine the relationship among DVA, JPE, and mVAS at the end of the study. The level of statistical significance was set at p < 0.05.
RESULTS
Fifteen participants were randomly allocated to the experimental group and fifteen were randomly allocated to the sham group. The demographic and baseline clinical characteristics of the two study groups are presented in Table 1. There were no significant differences between the two groups at baseline for gender, age, DVA, JPE, and mVAS. At the end of the study, there were significant differences in the mean DVA between the two groups (0.26±0.11 vs. 0.003±0.02, Table 2). Participants who were fatigued had a significantly poorer DVA than those who were not fatigued. There were, however, no significant differences in mean JPE between the two groups. Results showed that the DVA strongly correlated with the mVAS score (r = 0.79) No significant correlations were observed between the mVAS score and JPE (r = 0.23). A stepwise linear regression was conducted to determine the effect of JPE and mVAS on the cDVA score. The results indicated that mVAS was a significant predictor of DVA (R2 = 0.59). Approximately sixty percent of the variability in the DVA was explained by its relationship with the fatigue score.
Table 1. Mean (SD) of baseline characteristics for the participants in the experimental group and control group (N=30).
| Variables | Experimental (n=15) |
Control (n=15) |
|---|---|---|
| Gender+ | ||
| Male | 8 (53%) | 7 (47%) |
| Female | 7 (47%) | 8 (53%) |
| Age (years) | 25.3 (1.8) | 25.8 (2.4) |
| DVA | 0.06 (0.04) | 0.07 (0.03) |
| JPE | 4.54 (1.02) | 4.95 (1.25) |
| mVAS | 0.33 (0.7) | 0.20 (0.6) |
DVA: dynamic visual acuity, JPE: joint position error, mVAS: modified Visual Analog Scale. * χ2 test, ** Independent sample t-test, *** Mann-Whitney U-test, + Results are presented as frequencies (%)
Table 2. Comparison of the mean (SD) changes in post minus pre DVA and JPE between the two groups.
| Variables | Experimental (n=15) |
Control (n=15) |
|---|---|---|
| DVA (Analog Scale) | 0.26 (0.11) | 0.003 (0.02) |
| JPE (cm) | 0.97 (1.07) | 0.44 (1.40) |
DVA: dynamic visual acuity, JPE: joint position error, * Independent sample t-test
DISCUSSION
The purpose of the present investigation was to determine the effect of neck muscle fatigue on dynamic visual acuity in healthy young adults. Thirty young healthy participants were randomly assigned into either a neck muscle fatigue group or a sham group. Dynamic visual acuity test scores were used to measure the gaze stability, and JPE test scores were used to measure cervical joint repositioning accuracy. Our results determined that neck muscle fatigue negatively impacts DVA. Although not statistically significant, a trend was observed suggesting that neck muscle fatigue negatively impacts JPE, as determined by less accurate and less consistent repositioning performances in the fatigue group versus the sham group. Our findings are consistent with previous studies, which reported, that reduced proprioceptive acuity contributes to sensory mismatches and possibly an asymmetry of the VOR1, 14). This phenomenon is probably due to disturbances in the neural connections between the three sensory systems (somatosenory, vestibular, and vision) that can lead to mismatched sensory input, causing conflicts among all inputs from the different sensory systems1, 25,26,27,28,29). Moreover, our results demonstrated a strong positive linear relationship between DVA and neck muscle fatigue7, 30). This is likely because neck muscle fatigue has been shown in previous studies to modify the discharge of sensory receptors in neck muscles and affect proprioception1, 2, 4, 12). Consequently, neck muscle fatigue may affect the neural connections between the three sensory systems, which may be the main cause of the gain increase in VOR1, 14). Dorsal neck muscle fatigue induces postural instability, but not visual vertical misperception31). One study found no significant difference between with and without aframe in the road and frame test (RFT) when testing participants in a sitting position32). Thus, when assessing vestibular function in patients complaining of dizziness and/or visual disturbance with a history of neck trauma, one may speculate that VOR dysfunction could have a cervical origin due to somatosenory disturbance, which may lead to visual disturbances and dizziness. Recently, the rod and frame test (RFT) or tests measuring the perception of the subjective visual vertical (SVV) and subjective visual horizontal (SVH) have been used to study the functional effects of either neck pain or whiplash33). Furthermore, thoracic spine thrust manipulation proved effective in the treatment of individuals with neck pain, leading to a reduction in both pain and disability34). Another study reported that patients with mechanical neck pain who were treated with thoracic spine manipulation and exercise exhibited significantly greater improvements in disability in both short- and long-term follow-up periods35). However, we identified several limitations in the present study. Our study was conducted on healthy normal young adults. Future research should include healthy adults of various ages. Also, future studies should include patients with whiplash associated disorder (WAD) in order to measure the effects of neck trauma and pain on dynamic visual acuity. Finally, the effects of neck muscle fatigue on DVA should be compared in subjects in standing versus sitting. The results of this study suggest that neck muscle fatigue negatively impacts dynamic visual acuity. Although not statistically significant, cervical spine proprioception in the experimental group was diminished after fatigue as measured by the JPE. Clinical application of these findings suggests that patients with CGD may experience improved DVA and cervical proprioception through rehabilitation efforts directed at reducing cervical muscle fatigue.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia. The authors, therefore, acknowledge with thanks DSR technical and financial support.
REFERENCES
- 1.Sterling G, Treleaven DF, O’Leary S: Headache, and Neck Pain: Research-Based Directions for Physical Therapies, 1st ed. Edinburgh: Churchill Livingstone; 2008. [Google Scholar]
- 2.Kristjansson E, Treleaven J: Sensorimotor function and dizziness in neck pain: implications for assessment and management. J Orthop Sports Phys Ther, 2009, 39: 364–377. [DOI] [PubMed] [Google Scholar]
- 3.Karlberg M, Magnusson M, Malmström EM, et al. : Postural and symptomatic improvement after physiotherapy in patients with dizziness of suspected cervical origin. Arch Phys Med Rehabil, 1996, 77: 874–882. [DOI] [PubMed] [Google Scholar]
- 4.Wrisley DM, Sparto PJ, Whitney SL, et al. : Cervicogenic dizziness: a review of diagnosis and treatment. J Orthop Sports Phys Ther, 2000, 30: 755–766. [DOI] [PubMed] [Google Scholar]
- 5.Gosselin G, Rassoulian H, Brown I: Effects of neck extensor muscles fatigue on balance. Clin Biomech (Bristol, Avon), 2004, 19: 473–479. [DOI] [PubMed] [Google Scholar]
- 6.Schieppati M, Nardone A, Schmid M: Neck muscle fatigue affects postural control in man. Neuroscience, 2003, 121: 277–285. [DOI] [PubMed] [Google Scholar]
- 7.Pinsault N, Vuillerme N: Degradation of cervical joint position sense following muscular fatigue in humans. Spine, 2010, 35: 294–297. [DOI] [PubMed] [Google Scholar]
- 8.Revel M, Andre-Deshays C, Minguet M: Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch Phys Med Rehabil, 1991, 72: 288–291. [PubMed] [Google Scholar]
- 9.Heikkilä H, Aström PG: Cervicocephalic kinesthetic sensibility in patients with whiplash injury. Scand J Rehabil Med, 1996, 28: 133–138. [PubMed] [Google Scholar]
- 10.Heikkilä HV, Wenngren BI: Cervicocephalic kinesthetic sensibility, active range of cervical motion, and oculomotor function in patients with whiplash injury. Arch Phys Med Rehabil, 1998, 79: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 11.Vuillerme N, Pinsault N, Vaillant J: Postural control during quiet standing following cervical muscular fatigue: effects of changes in sensory inputs. Neurosci Lett, 2005, 378: 135–139. [DOI] [PubMed] [Google Scholar]
- 12.Stapley PJ, Beretta MV, Dalla Toffola E, et al. : Neck muscle fatigue and postural control in patients with whiplash injury. Clin Neurophysiol, 2006, 117: 610–622. [DOI] [PubMed] [Google Scholar]
- 13.Tjell C, Rosenhall U: Smooth pursuit neck torsion test: a specific test for cervical dizziness. Am J Otol, 1998, 19: 76–81. [PubMed] [Google Scholar]
- 14.Padoan S, Karlberg M, Fransson PA, et al. : Passive sustained turning of the head induces asymmetric gain of the vestibulo-ocular reflex in healthy subjects. Acta Otolaryngol, 1998, 118: 778–782. [DOI] [PubMed] [Google Scholar]
- 15.Hikosaka O, Maeda M: Cervical effects on abducens motoneurons and their interaction with vestibulo-ocular reflex. Exp Brain Res, 1973, 18: 512–530. [DOI] [PubMed] [Google Scholar]
- 16.Nashner L: Computerized dynamic posturography. Clinical applications. In: Jacobson GP, Newman CW, Kartush JM eds., Handbook of Balance Function Testing. St. Louis: Mosby Year Book Inc., 2000, pp 308–333. [Google Scholar]
- 17.Szentkirályi A, Csilla Z, Madarász, Márta Novák: Sleep disorders: impact on daytime functioning and quality of life. Expert Rev Pharmacoeconomics Outcomes Res, 2009, 9: 49–64. [DOI] [PubMed] [Google Scholar]
- 18.Instructions for Use: inVision™, System operator’s manual, Version 8.1. Clackamas: NeuroCom® International Inc., 2004.
- 19.Herdman SJ, Tusa RJ, Blatt P, et al. : Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am J Otol, 1998, 19: 790–796. [PubMed] [Google Scholar]
- 20.Herdman SJ: Computerized dynamic visual acuity test in the assessment of vestibular deficits. In: Scott DZE, David SZ, eds. Handbook of Clinical Neurophysiology, 1st ed. Elsevier, 2010, pp 181–190. [Google Scholar]
- 21.Roren A, Mayoux-Benhamou MA, Fayad F, et al. : Comparison of visual and ultrasound based techniques to measure head repositioning in healthy and neck-pain subjects. Man Ther, 2009, 14: 270–277. [DOI] [PubMed] [Google Scholar]
- 22.Pinsault N, Fleury A, Virone G, et al. : Test-retest reliability of cervicocephalic relocation test to neutral head position. Physiother Theory Pract, 2008, 24: 380–391. [DOI] [PubMed] [Google Scholar]
- 23.Pinsault N, Vuillerme N, Pavan P: Cervicocephalic relocation test to the neutral head position: assessment in bilateral labyrinthine-defective and chronic, nontraumatic neck pain patients. Arch Phys Med Rehabil, 2008, 89: 2375–2378. [DOI] [PubMed] [Google Scholar]
- 24.Borg G: Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health, 1990, 16: 55–58. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC: Textbook of Medical Physiology, 8th ed. Philadelphia: W.B. Saunders Company, 1991, pp 67–79. [Google Scholar]
- 26.Corneil BD, Olivier E, Munoz DP: Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol, 2002, 88: 1980–1999. [DOI] [PubMed] [Google Scholar]
- 27.Dutia MB: The muscles and joints of the neck: their specialisation and role in head movement. Prog Neurobiol, 1991, 37: 165–178. [DOI] [PubMed] [Google Scholar]
- 28.Jull G, Falla D, Treleavan J: therapeutic exercise approach for cervical disorders. In: Boyling JD, Jull G, eds. Grieve’s Modern Manual Therapy. The Vertebral Column. Edinburgh: Churchill Livingstone, 2004, pp 451–461. [Google Scholar]
- 29.Revel M, Minguet M, Gregoy P, et al. : Changes in cervicocephalic kinesthesia after a proprioceptive rehabilitation program in patients with neck pain: a randomized controlled study. Arch Phys Med Rehabil, 1994, 75: 895–899. [DOI] [PubMed] [Google Scholar]
- 30.Vuillerme N, Pinsault N, Bouvier B: Cervical joint position sense is impaired in older adults. Aging Clin Exp Res, 2008, 20: 355–358. [DOI] [PubMed] [Google Scholar]
- 31.Panichaporn W, Hiengkaew V, Thanungkul S, et al. : Postural stability and visual verticality perception of neck disturbance of the middle-aged during quiet standing. J Phys Ther Sci, 2013, 25: 281–285. [Google Scholar]
- 32.Bagust J: Assessment of verticality perception by a rod-and-frame test: preliminary observations on the use of a computer monitor and video eye glasses. Arch Phys Med Rehabil, 2005, 86: 1062–1064. [DOI] [PubMed] [Google Scholar]
- 33.Grod JP, Diakow PR: Effect of neck pain on verticality perception: a cohort study. Arch Phys Med Rehabil, 2002, 83: 412–415. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira L, Santos L, Neto W, et al. : Analysis of thoracic spine thrust manipulation for reducing neck pain. J Phys Ther Sci, 2013, 25: 325–329. [Google Scholar]
- 35.Kanchanomai S, Janwantanakul P, Jiamjarasrangsi W: One-year incidence and risk factors of thoracic spine pain in undergraduate students. J Phys Ther Sci, 2013, 25: 15–20. [Google Scholar]