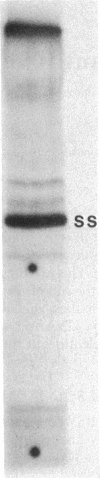
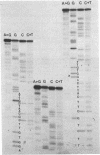
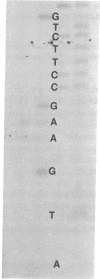
Abstract
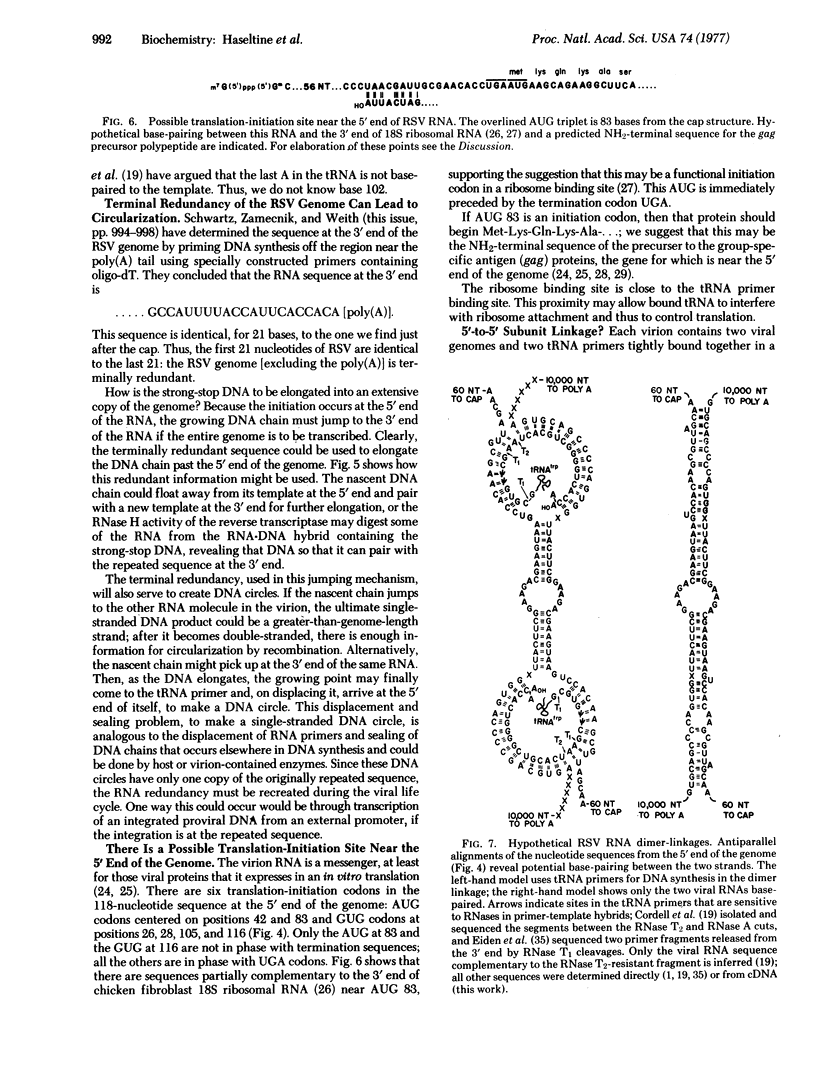
When Rous sarcoma virus RNA is transcribed into DNA by the reverse transcriptase, a tRNA primer is elongated into DNA. The primer is near the 5' end of the virus genome; the first major DNA made is a "run-off" product extending 101 bases from the primer to the 5' end of the template. We have studied this DNA molecule to determine the sequence of the first 101 bases at the 5' end of the Rous sarcoma virus genome (Prague strain, subgroup C). Twenty-one bases at the extreme 5' end are also at the 3' end of the virus genome (see D. E. Schwartz, P. C. Zamecnik, and H. L. Weith, this issue, pp. 994-998), and thus this virus is terminally redundant. The existence of this sequence repetition immediately suggests mechanisms by which the growing DNA copy can jump from the 5' end to a 3' end of the template and become circular. The sequence also displays a possible ribosome binding site and enough secondary structure to permit a possible 5'-5' linkage of viral RNA molecules.
Full text
PDF
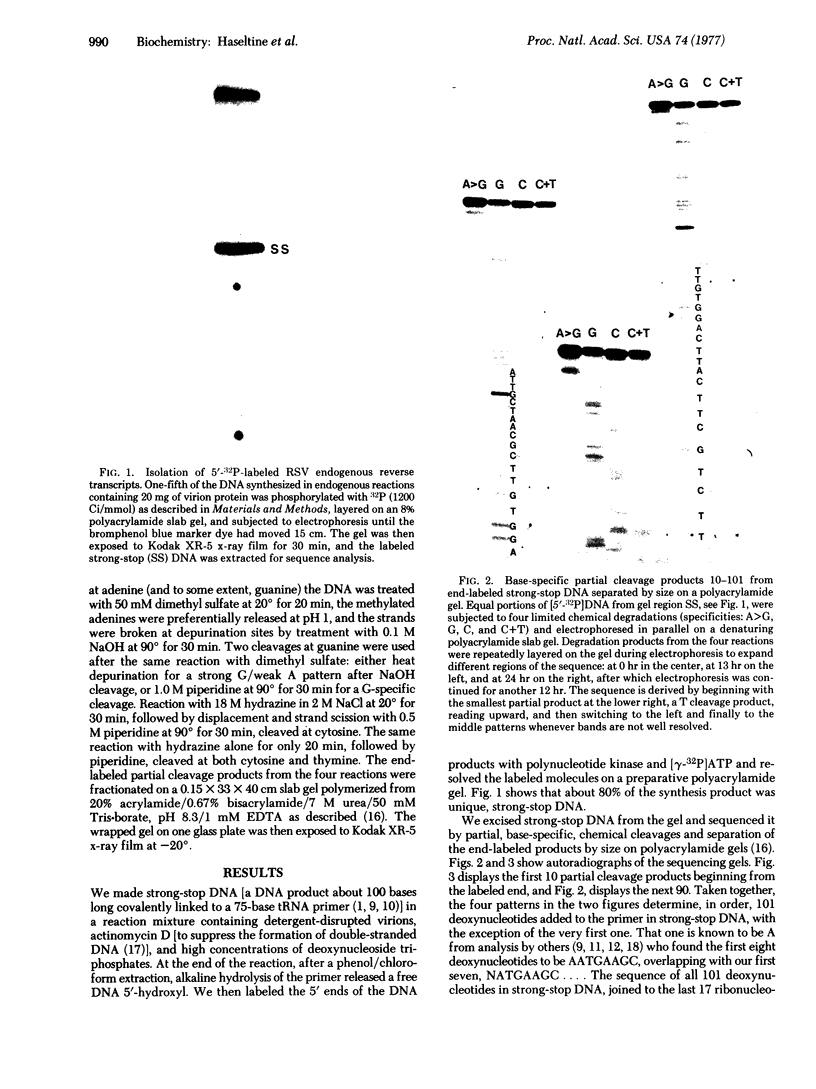


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Bigger C. H., Murray K., Murray N. E. Recognition sequence of a restriction enzyme. Nat New Biol. 1973 Jul 4;244(131):7–10. doi: 10.1038/newbio244007a0. [DOI] [PubMed] [Google Scholar]
- Cashion L. M., Joho R. H., Planitz M. A., Billeter M. A., Weissmann C. Initiation sites of Rous sarcoma virus RNA-directed DNA synthesis in vitro. Nature. 1976 Jul 15;262(5565):186–190. doi: 10.1038/262186a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of DNA from the 70S RNA of Rous sarcoma virus: identification and characterization of various size classes of DNA transcripts. J Virol. 1975 Nov;16(5):1220–1228. doi: 10.1128/jvi.16.5.1220-1228.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Quade K., Nichols J. L. Interaction of tryptophan transfer RNA with Rous sarcoma virus 35S RNA. Nature. 1976 Jan 22;259(5540):245–247. doi: 10.1038/259245a0. [DOI] [PubMed] [Google Scholar]
- Eladari M. E., Galibert F. Sequence determination of the 3' terminal T1 oligonucleotide of 18S ribosomal RNA. Nucleic Acids Res. 1976 Oct;3(10):2749–2755. doi: 10.1093/nar/3.10.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith J., Fraenkel-Conrat H. Identification of the 5' end of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3347–3350. doi: 10.1073/pnas.72.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleid D., Humayun Z., Jeffrey A., Ptashne M. Novel properties of a restriction endonuclease isolated from Haemophilus parahaemolyticus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):293–297. doi: 10.1073/pnas.73.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Hu S., Bender W., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell. 1976 Apr;7(4):609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Identical 3'-terminal octanucleotide sequence in 18S ribosomal ribonucleic acid from different eukaryotes. A proposed role for this sequence in the recognition of terminator codons. Biochem J. 1974 Sep;141(3):609–615. doi: 10.1042/bj1410609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cordell-Stewart B., Rohde W., Goodman H. M., Bishop J. M. Reassociation of 4 S and 5 S RNA's with the genome of avian sarcoma virus. Virology. 1975 May;65(1):248–259. doi: 10.1016/0042-6822(75)90025-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Garfin D. E., Levinson W. E., Bishop J. M., Goodman H. M. Tumor virus ribonucleic acid directed deoxyribonucleic acid synthesis: nucleotide sequence at the 5' terminus of nascent deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3159–3163. doi: 10.1021/bi00712a024. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]