Abstract
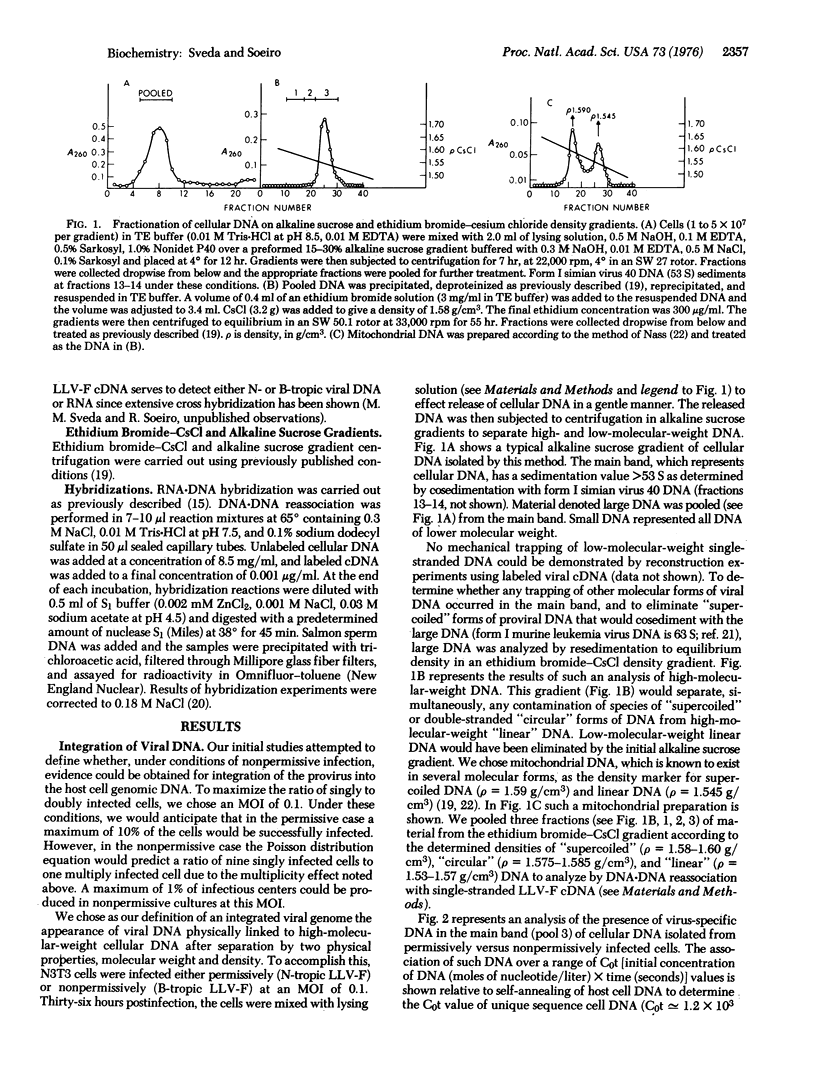
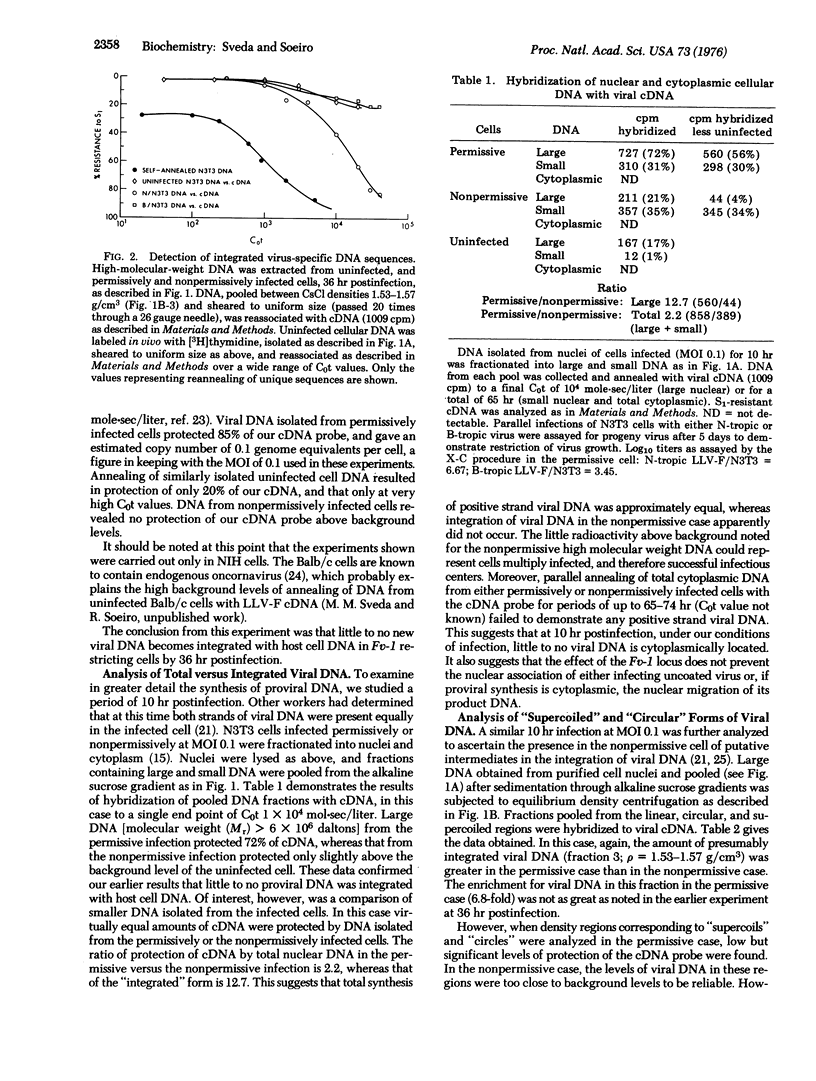
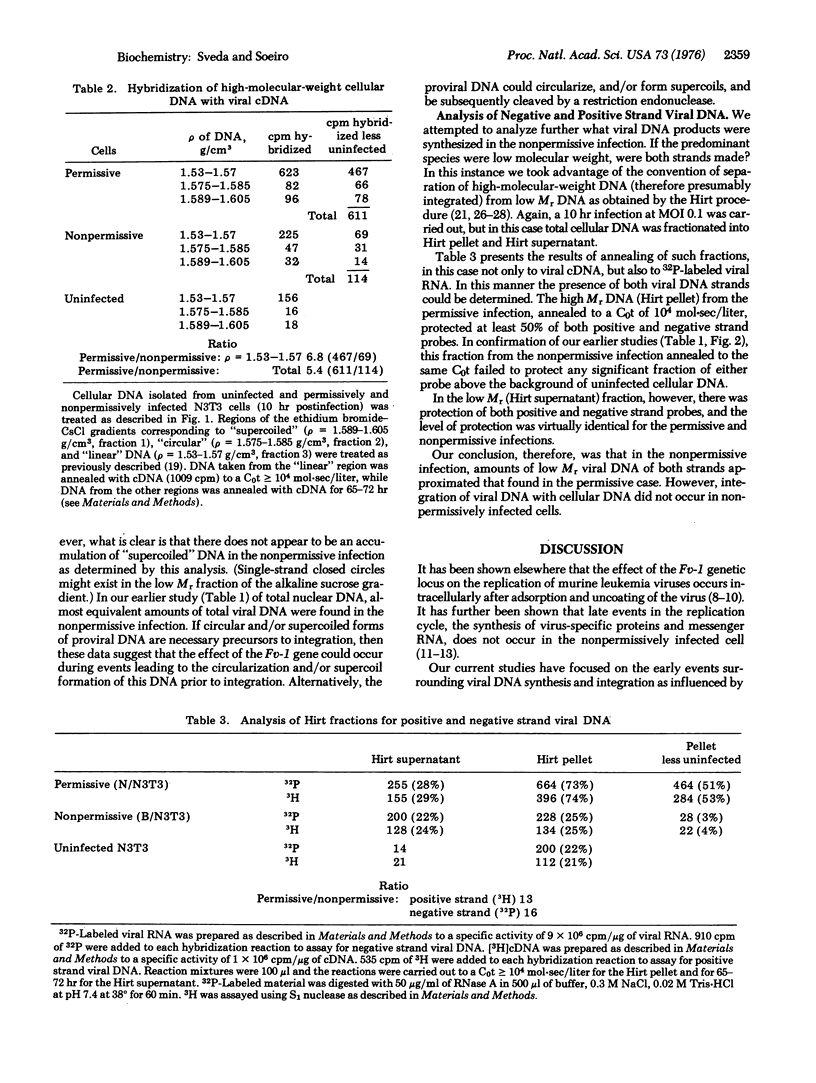
Host restriction of exogenous infection by murine leukemia viruses is controlled in vitro predominantly by the murine Fv-1 locus. The mechanism of this host restriction was investigated by comparing the early events in the replication of N-tropic versus B-tropic Friend leukemia virus in NIH 3T3 cells. These cells, which are Fv-1nn in type, are permissive for the N-tropic strain, but nonpermissive for the B-tropic strain, which replicates permissively in Balb/c cells. We have studied the synthesis, intracellular location, and molecular form of virus-specific DNA early in replication by means of molecular hybridization with a virus-specific DNA probe. Our results suggest that in the permissive infection viral DNA rapidly becomes integrated with cellular DNA. However, in the nonpermissive infection, although almost equal amounts of both positive and negative strand viral DNA are synthesized, integration of the provirus does not occur.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Differential cellular regulation of three distinct classes of type C RNA viruses endogenous to mouse cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1129–1137. doi: 10.1101/sqb.1974.039.01.129. [DOI] [PubMed] [Google Scholar]
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Gerwin B. I., Duran-Troise G., Gisselbrecht S., Rein A. Murine sarcoma virus pseudotypes acquire a determinant specifying N or B tropism from leukaemia virus during rescue. Nature. 1975 Jul 17;256(5514):223–225. doi: 10.1038/256223a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Declève A., Niwa O., Gelmann E., Kaplan H. S. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology. 1975 Jun;65(2):320–332. doi: 10.1016/0042-6822(75)90038-0. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on synthesis of N-tropic and B-tropic murine leukemia viral RNA. Cell. 1976 Jan;7(1):33–39. doi: 10.1016/0092-8674(76)90252-x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of the Fv-1 locus on the titration of murine leukemia viruses. J Virol. 1975 Dec;16(6):1593–1598. doi: 10.1128/jvi.16.6.1593-1598.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Markham P. D., Baluda M. A. Integrated state of oncornavirus DNA in normal chicken cells and in cells transformed by avian myeloblastosis virus. J Virol. 1973 Oct;12(4):721–732. doi: 10.1128/jvi.12.4.721-732.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. II. Structure and physicochemical properties of isolated DNA. J Mol Biol. 1969 Jun 28;42(3):529–545. doi: 10.1016/0022-2836(69)90241-1. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray U., Soeiro R., Fields B. N. Host restriction of Friend Leukemia virus coat protein synthesis. J Virol. 1976 Apr;18(1):370–373. doi: 10.1128/jvi.18.1.370-373.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Kashmiri S. V., Bassin R. H., Gerwin B. L., Duran-Troise G. Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell. 1976 Mar;7(3):373–379. doi: 10.1016/0092-8674(76)90166-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Fields B. N., Soeiro R. Fate of input oncornavirion RNA--biological studies. J Virol. 1976 Apr;18(1):85–91. doi: 10.1128/jvi.18.1.85-91.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Fields B. N., Soeiro R. Host restriction of friend leukemia virus; fate of input virion RNA. Cell. 1974 Aug;2(4):271–277. doi: 10.1016/0092-8674(74)90021-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Yoshikura H. Host range conversion of the murine sarcoma-leukaemia complex. J Gen Virol. 1973 Jun;19(3):321–327. doi: 10.1099/0022-1317-19-3-321. [DOI] [PubMed] [Google Scholar]



