Abstract
AIM: To evaluate the correlation between CD4, CD8 cell infiltration in gastric mucosa, Helicobacter pylori (H pylori) infection and symptoms or the assemblage of symptoms in cases with chronic gastritis.
METHODS: Biopsy samples at the gastric antrum were obtained from 62 patients with chronic gastritis. CD4 and CD8 cell infiltration was evaluated by immunohistochemical assays on frozen sections of the biopsy samples. Fifteen symptoms referring to digestion-related activity and non-digestion related activity were observed. The correlation between lymphocyte infiltration and each symptom or symptom assemblage was analyzed by logistic regression and K-mean cluster methods.
RESULTS: CD4 cell infiltrations in gastric mucosa were much more in patients with H pylori infection, while CD8 cell infiltrations were similar in patients with or without H pylori infection. Logistic regression analysis showed that the symptoms including heavy feeling in head or body (t = 2.563), and thirst (t = 2.478) were significantly related with CD4 cell infiltration in gastric mucosa (P<0.05), and cool limbs with aversion to cold were related with CD8 cell infiltration (t = 2.872, P<0.05). Further analysis showed that non-digestive related symptom assemblage could increase the predicted percentage of CD4 and CD8 cell infiltration in gastric mucosa, including lower CD4 infiltration by 12.5%, higher CD8 infiltration by 33.3%, and also non-H pylori infection by 23.6%. K-means cluster analysis of all symptoms and CD4 and CD8 cell infiltration in gastric mucosa showed a similar tendency to increase the predicted percentage of CD4, CD8 cell infiltration and H pylori infection.
CONCLUSION: Based on correlation between the gastric mucosa lymphocyte infiltration, H pylori infection and clinical symptoms, symptoms or symptomatic assemblages play an important role in making further classification of chronic gastritis, which might help find a more specific therapy for chronic gastritis.
Keywords: Mucosal immune, Helicobacter pylori infection, Symptoms, Chronic gastritis
INTRODUCTION
The role of immune reactions in Helicobacter pylori (H pylori) infection and chronic gastritis is a research area of rapid progress[1,2]. It has been recognized that lamina propria lymphocytes are essential in gastric lesions induced by H pylori infection[3-5]. However, the correlation concerning H pylori infection and CD4 and CD8 lymphocytes in gastric mucosa is not well understood. Moreover, the lack of cognition for the complex manifestations of chronic gastritis is another nodus for the effect of subjective symptoms on the objective pathologic parameters, which may lead to a further diagnostic classification of chronic gastritis.
To further explore the correlation between the H pylori infection and CD4 and CD8 cell infiltration in gastric mucosa in patients with chronic gastritis, a clinical investigation was designed and a novel analytical method was proposed in this work. More importantly, the study based on the important role of subjective symptoms in disease identification in traditional Chinese medicine (TCM), took a different perspective in assessing the association between clinical subjective manifestations and objective parameters including H pylori infection, CD4 and CD8 cell infiltration in gastric mucosa.
MATERIALS AND METHODS
Patients
A total of 62 patients with chronic gastritis, who were diagnosed through gastroscopy and mucosal biopsy, were included in the present study. All patients were investigated by the Beijing Traditional Chinese Medicine Hospital in 2002. Among them, 29 were males and 33 were females, aged from 18 to 65 years with a mean age of 42 years. Gastric biopsies were histologically evaluated for chronic gastritis diagnosis according to the criteria of the visual analog scale in Sydney classification and grading of gastritis[6], and immunohistologically evaluated for CD4 and CD8 cell infiltrations[7].
Diagnosis of H pylori infection
Three specimens of gastric mucosa were obtained from each patient via endoscopy. Gastric mucosa was sampled from the area of greater curvature at gastric antrum, H pylori infection was determined by pathological staining with hematoxylin and eosin (HE) followed by Giemsato. Under a microscope, H pylori could be observed as a typical curve like S. It looked like a short bacillus or a globular body with a slight curve.
Detection of CD4 and CD8 cells in gastric mucosa
Gastric mucosa tissues were sampled by gastric mucosal biopsy from the antrum of each patient. Immunohistochemical assay was used to detect the infiltration of CD4 and CD8 cells in gastric mucosa frozen sections. The test kits were from Vector Laboratory (Vector Stain ABC Kits). Briefly, for the detection of CD4 and CD8 cells, the first antibodies were rabbit anti-human antibodies, and the second antibodies were goat anti-rabbit antibodies, and the samples were stained with DAB (Sigma, USA). Positive granules could be observed. The average of positive granules of three samples from Q-win DC100 image analysis was used for further statistical analysis.
Symptom observation
Fifteen common clinical symptoms based on TCM were observed as follows. Digestion-related symptoms included stomachache, distending fullness in stomach, abdomen, nausea or vomit, acid regurgitation and epigastric upset, diarrhea, hard stool, and constipation. Non-digestion related symptoms included weakness of body or faint limbs, lower spirit, heavy feeling in head or whole body, irritating, distending fullness in the chest, thirst, weak taste without thirst, cool limbs with aversion to cold. The symptoms observed on the day of biopsy were taken for the analysis.
Statistical analysis
SPSS 11.5 statistical package program was used for data analysis. The variables were processed by ANVOA analysis and logistic regression analysis, respectively. The clusters of CD4, CD8 cell infiltrations and symptoms were analyzed by k-means cluster method for further ANOVA and logistic regression analyses.
RESULTS
Table 1 shows that CD4 cell infiltrations in gastric mucosa was much more in the cases with H pylori infection, while CD8 cell infiltrations were similar in patients with or without H pylori infection (Figure 1). The results suggested that CD4 cell infiltrations were positively related with H pylori infection.
Table 1.
Changes CD4 and CD8 cells in H pylori positive and negative cases (mean±SD).
| HP infection | n | CD4 | CD8 |
| Positive | 45 | 9102.82±2747.18a | 6285.67±3308.74 |
| Negative | 17 | 6255.33±3284.88 | 6992.91±3524.89 |
P<0.05 vs negative cases.
Figure 1.
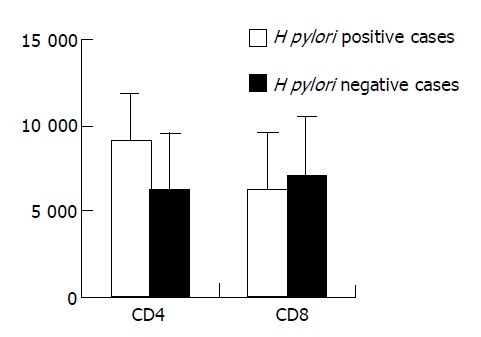
Difference of CD4 and CD8 cell infiltrations in gastric mucosa between H pylori positive and negative cases (P<0.05).
Table 2 shows the importance of each symptom in CD4 cell infiltrations in gastric mucosa (logistic regression). It demonstrated that the heavy feeling of head or body and thirst were significantly related with CD4 cell infiltrations (P<0.05).
Table 2.
Correlation between symptoms and CD4 cell infiltrations.
| P | Symptom |
| P = 0.01-0.05 | Heavy head or heavy body (t = 2.563), thirst (t = 2.478) |
| P = 0.06-0.2 | Lower spirit, weakness of body or faint limbs, irritation |
| P = 0.21-0.5 | Stomachache, hard stool, weak taste without thirst, distending fullness in chest, cool limbs with aversion to cold |
| P>0.6 | Distending fullness in abdomen, diarrhea, constipation, acid regurgitation and epigastric upset, nausea or vomiting |
Table 3 shows the importance of each symptom in CD8 cell infiltrations in gastric mucosa (logistic regression). It demonstrated that the cool limbs with aversion to cold were significantly related with CD8 cell infiltrations (P<0.05).
Table 3.
Correlation between symptoms and CD8 cell infiltrations.
| P | Symptom |
| P = 0.01-0.05 | Cool limbs with aversion to cold (t = 2.872) |
| P = 0.06-0.2 | Stomachache, nausea or vomiting |
| P = 0.21-0.5 | Weakness of body or faint limbs |
| P>0.6 | Heavy feeling of head or body, thirst, distending fullness in abdomen, diarrhea, constipation, acid regurgitation and epigastric upset, hard stool, weak taste without thirst, distending fullness in chest, lower spirit, irritation |
Referring to the results in Tables 2 and 3, the non-digestion related symptoms might have positive relations with CD4 and CD8 cell infiltrations in gastric mucosa. Thus, the symptoms were classified into two groups of digestion and non-digestion related symptoms based on the common clinical results, and then the data were analyzed with logistic regression for the relationship between the assemblage of symptoms, clusters of CD4 or CD8 cell infiltrations, and H pylori infection (Tables 4 and 5).
Table 4.
Effect of symptom assemblages on the predicted percentage of CD4 and CD8 cell infiltrations.
| Symptom | Cell level | Predicted percentage of CD4 | Predicted percentage of CD8 |
| Digestion related | Lower | 62.5 | 89.2 |
| Higher | 62.1 | 16.7 | |
| Overall | 62.3 | 60.7 | |
| Non-digestion related | Lower | 75.0 | 83.8 |
| Higher | 58.6 | 50.0 | |
| Overall | 67.2 | 70.5 |
Table 5.
Effect of symptom assemblages on the predicted percentage of H pylori infection.
| Symptom | H pylori infection | Predicted percentage |
| Digestion related | Negative | 17.6 |
| Positive | 95.5 | |
| Overall | 73.8 | |
| Non-digestion related | Negative | 41.2 |
| Positive | 93.2 | |
| Overall | 78.7 |
Table 4 shows that the non-digestion related symptom assemblage could increase the predicted percentage of CD4 cell infiltrations. Lower CD4 infiltration was increased by 12.5%, higher CD4 infiltration was increased by 3.5% and the total increase was 5.1%. Table 4 also shows that the non-digestion related symptom assemblage could affect the predicted percentage of CD8 cell infiltrations. Higher CD8 infiltration was increased 33.3% and the total increase was 9.8%, and lower CD8 infiltration was decreased 5.4%. The results suggested that the non-digestion related symptom assemblage might have positive relations with CD4 and CD8 cell infiltrations in gastric mucosa.
Table 5 shows that the non-digestive related symptom assemblage could increase the predicted percentage of patients with or without H pylori infection. It was increased 23.6% in patients without H pylori infection and the total increase was 4.9%, and was similar in patients with H pylori infection.
In order to further explore the importance of symptom assemblages in CD4 and CD8 cell infiltrations in gastric mucosa, the cases were classified into two categories via K-mean cluster analysis according to the clinical manifestations, and then the relationship between the two categories of symptoms and CD4, CD8 cell infiltrations was analyzed by logistic regression method (Table 6).
Table 6.
Effect of symptom assemblage based on K-mean cluster analysis on the predicted percentage of CD4 and CD8 infiltrations and H pylori infection.
| Symptom | Infiltration | Predicted percentage of CD4 | Predicted percentage of CD8 | H pylori infection | Predicted percentage |
| Category one1 | Lower | 78.1 | 100 | Negative | 0 |
| Higher | 44.8 | 0 | Positive | 100 | |
| Overall | 62.3 | 60.7 | Overall | 72.1 | |
| Category two1 | Lower | 68.8 | 81.1 | Negative | 35.3 |
| Higher | 62.1 | 58.3 | Positive | 90.9 | |
| Overall | 65.6 | 72.1 | Overall | 75.4 |
Category one included distending fullness in abdomen, stomachache, diarrhea, regurgitation and epigastric upset, and category two had other symptoms.
Table 6 shows that the digestion related symptoms including distending fullness in abdomen, stomachache, diarrhea, regurgitation and epigastric upset were clustered into category 1, and the others including all non-digestion related symptoms were clustered into category 2. The symptom assemblage in category 2 could affect the predicted percentage of CD4 cell infiltration. Lower CD4 infiltration was increased 9.3%, higher CD4 infiltration was increased 17.3% and the total increase was 2.3%. Table 6 also shows that the symptom assemblage in category 2 could affect the predicted percentage of CD8 cell infiltration. Lower CD8 was decreased 9.9%, higher CD8 was increased 58.3% and the total increase was 11.4%. Table 6 also shows that the symptom assemblage in category 2 could affect the predicted percentage of H pylori infection. It was increased by 35.5% in patients without H pylori infection and the total increase was 3.3%, and was similar in patients with H pylori infection.
The results in Tables 4-6 further suggested that there might be a positive relationship between the subjective manifestations and the objective parameters (CD4 and CD8 cell infiltrations). To further explore their relationships in chronic gastritis, all cases were classified into two categories via K-mean cluster analysis based on the symptoms, CD4 and CD8 cell infiltrations, and then the symptom differences between the two clusters of patients were analyzed by ANOVA.
Table 7 shows the importance of each symptom in the two clusters. It showed that the weakness of body or faint limbs, lower spirit, hard stool, played a significant role in the cluster identification (P<0.05) while constipation, thirst, distending fullness in chest, nausea or vomiting, had a potential role in the two cluster identification (P = 0.06-0.2).
Table 7.
Difference of symptoms between the two clusters of cases based on the symptoms, CD4 and CD8 cell infiltrations.
| P | Symptom |
| P = 0.01-0.05 | Weakness of body or faint limbs (F = 5.005), lower spirit (F = 5.750), hard stool (F = 5.835) |
| P = 0.06-0.2 | Constipation, thirst, distending fullness in chest, nausea or vomiting |
| P = 0.21-0.5 | Stomachache, diarrhea, weak taste without thirst, cool limbs with aversion to cold |
| P>0.6 | Heavy feeling of head or body, distending fullness in abdomen, acid regurgitation and epigastric upset, irritation |
The results in Table 7 were similar to those in Tables 2-6, and further suggested that the non-digestion related symptoms or symptom assemblage were positively related with CD4 and CD8 cell infiltrations.
DISCUSSION
Chronic gastritis is related to H pylori infection, which may cause immunological reactions in peripheral mononuclear cells. The activity and characteristics of peripheral mononuclear cells may differ in ulcer and non-ulcer patients infected with H pylori[3]. It has been reported that CD4 cells are sensitized in vivo and migrate to gastric mucosa where they induce gastritis in response to H pylori antigens, suggesting that CD4-dependent H pylori gastritis could lead to epithelial damage with proliferative and metaplastic responses[4,5,7]. The number of activated cytotoxic lamina propria lymphocytes was increased in gastric mucosa affected with acute gastric mucosal lesions, suggesting that lymphocytes are crucial in the pathogenesis of gastric lesions[8-10]. Yuceyar et al[11], found that there was no obvious alteration in total T and B lymphocytes and CD4+ T, CD8+ T lymphocytes and natural killer cells in chronic antral gastritis patients compared to normal persons, suggesting that there is no systemic alteration in the specific immune system in response to H pylori in patients with chronic antral gastritis[11]. Itoh et al[12], found that gastric T cells were differentiated to produce a large amount of IFN-gamma by a mechanism unrelated to H pylori infection. H pylori infection appeared to activate T cells to secrete even more IFN-γ, which might contribute to maintaining a perpetual inflammation in H pylori-infected stomach. Our results showed that CD4 cells in gastric mucosa were much more in patients with H pylori infection, while CD8 cells were similar in patients with or without H pylori infection (Table 1). However, the mechanism how CD4 cells affect gastric mucosal lesions remains unclear.
The clinical manifestations of CG patients are intricate. They are divergent due to the pathological changes of gastric mucosa, and are affected by environmental factors. However, the relationship between the divergent manifestations and pathological changes is not completely understood. The study on chronic gastritis has shown that proper assemblages of symptoms could improve the accuracy of H pylori infection classification, and improper assemblages could decrease the accuracy of H pylori infection classification. Our results in this paper showed that the symptoms including the heavy feeling of head or body and thirst were significantly related with CD4 cell distributions in gastric mucosa (P<0.05), and cool limbs with aversion to cold were related with CD8 cells (P<0.05). Also the symptoms including lower spirit, weakness of body or faint limbs and irritation were related with CD4 cell distributions (P = 0.06-0.2), while stomachache, nausea or vomiting were related with CD8 cells (P=0.06-0.2), suggesting that the different objective symptoms might play different roles in H pylori infection and lymphocyte infiltrations in gastric mucosa. Further logistic analysis showed that non-digestion related symptom assemblage could increase the predicted percentage of CD4 and CD8 cells in gastric mucosa, and the percentage of non-H pylori infection to some degree (Tables 2 and 3). Also the symptom assemblages classified with K-mean cluster analysis showed similar results in predicting the percentage of H pylori infection, lymphocyte infiltration (Tables 4 and 5). The results showed that the proper combination of symptoms might play a more important role in predicting the percentage of H pylori infection and lymphocyte infiltration. To further explore the contribution of symptom assemblages to CD4 and CD8 cell distributions in gastric mucosa, the cases were classified into two categories via K-mean cluster analysis according to the clinical manifestations, and then the relationship between the two categories of symptoms and CD4, CD8 cells was analyzed by logistic regression method. The results showed that digestion-related symptoms including distending fullness in abdomen, stomachache, diarrhea, regurgitation and epigastric upset were clustered into category 1, and the others including all non-digestion related symptoms were clustered into category 2. The symptom assemblage in category 2 could affect the predicted percentage of lymphocyte infiltration in gastric mucosa and H pylori infection (Table 6). Our further analysis on the two cluster symptoms via ANOVA showed that the symptoms, such as weakness of body or faint limbs, lower spirit, hard stool, played a significant role in classification of the two symptom assemblages (P<0.05), and the results further suggested that different symptoms might play different roles in H pylori infection and lymphocyte infiltration in gastric mucosa, and that the non-digestion related symptoms played a more important role in H pylori infection and lymphocyte infiltration in gastric mucosa.
Similar results on the positive relationship between subjective symptoms or symptom assemblages and objective parameter such as pathological changes and therapeutic effects, are described in detail in TCM. The identification of diseases (Zheng identification, or Zheng differentiation) in TCM depends on the information obtained from the interrogation, auscultation, inspection and pulse-feeling, and the major characteristics of the information are its subjectivity. The long history of TCM has proven that the subjective symptoms play a more important role in the diagnosis and treatment of diseases. Our results indicate that further studies on the relationship between subjective symptoms and objective parameters are needed.
In conclusion, the symptoms or symptomatic assemblages have a positive correlation with CD4, CD8 cells and H pylori infection, and might play an important role in making further classification of chronic gastritis, which might help to find a more specific therapy for different groups of chronic gastritis.
Footnotes
Supported by the National Science Foundation, China, No. 90209002 and 90209032; Key Grant from National Administration of Traditional Chinese Medicine, No. 000-J-Z-02; Beijing Creative Human Resource Plan
Science Editor Wang XL Language Editor Elsevier HK
References
- 1.Aguilar GR, Ayala G, Fierros-Zárate G. Helicobacter pylori: recent advances in the study of its pathogenicity and prevention. Salud Publica Mex. 2001;43:237–247. doi: 10.1590/s0036-36342001000300010. [DOI] [PubMed] [Google Scholar]
- 2.Prinz C, Schöniger M, Rad R, Becker I, Keiditsch E, Wagenpfeil S, Classen M, Rösch T, Schepp W, Gerhard M. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res. 2001;61:1903–1909. [PubMed] [Google Scholar]
- 3.Ohara T, Arakawa T, Higuchi K, Kaneda K. Overexpression of co-stimulatory molecules in peripheral mononuclear cells of Helicobacter pylori-positive peptic ulcer patients: possible difference in host responsiveness compared with non-ulcer patients. Eur J Gastroenterol Hepatol. 2001;13:11–18. doi: 10.1097/00042737-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Peterson RA, Hoepf T, Eaton KA. Adoptive transfer of splenocytes in SCID mice implicates CD4+ T cells in apoptosis and epithelial proliferation associated with Helicobacter pylori-induced gastritis. Comp Med. 2003;53:498–509. [PubMed] [Google Scholar]
- 5.Riedel S, Kraft M, Kucharzik T, Pauels HG, Tiemann M, Steinbüchel A, Domschke W, Lügering N. CD4+ Th1-cells predominate in low-grade B-cell lymphoma of gastric mucosa-associated lymphoid tissue (MALT type) Scand J Gastroenterol. 2001;36:1198–1203. doi: 10.1080/00365520152584842. [DOI] [PubMed] [Google Scholar]
- 6.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Sommer F, Faller G, Konturek P, Kirchner T, Hahn EG, Zeus J, Röllinghoff M, Lohoff M. Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–5546. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Ito M, Hayasaki N, Ishihara A, Ando T, Ina K, Kusugami K. Cytotoxic molecules expressed by intraepithelial lymphocytes may be involved in the pathogenesis of acute gastric mucosal lesions. J Gastroenterol. 2003;38:216–221. doi: 10.1007/s005350300039. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K, Higuchi K, Arakawa T, Kobayashi K, Kaneda K. Phenotypical and morphological analyses of intraepithelial and lamina propria lymphocytes in normal and regenerating gastric mucosa of rats in comparison with those in intestinal mucosa. Arch Histol Cytol. 2000;63:159–167. doi: 10.1679/aohc.63.159. [DOI] [PubMed] [Google Scholar]
- 10.De Silva HD, Van Driel IR, La Gruta N, Toh BH, Gleeson PA. CD4+ T cells, but not CD8+ T cells, are required for the development of experimental autoimmune gastritis. Immunology. 1998;93:405–408. doi: 10.1046/j.1365-2567.1998.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuceyar H, Saruc M, Kokuludag A, Terzioglu E, Goksel G, Isisag A. The systemic cellular immune response in the Helicobacter pylori-associated duodenal ulcer and chronic antral gastritis. Hepatogastroenterology. 2002;49:1177–1179. [PubMed] [Google Scholar]
- 12.Itoh T, Wakatsuki Y, Yoshida M, Usui T, Matsunaga Y, Kaneko S, Chiba T, Kita T. The vast majority of gastric T cells are polarized to produce T helper 1 type cytokines upon antigenic stimulation despite the absence of Helicobacter pylori infection. J Gastroenterol. 1999;34:560–570. doi: 10.1007/s005350050373. [DOI] [PubMed] [Google Scholar]


