Abstract
AIM: Most studies on the immune effect of gp96 were focused on its enhancement of CTLs. It is interesting to know whether gp96 could influence the humoral immune response, and whether the recombinant N-terminal fragment of gp96 could substitute native gp96 to stimulate the immune system.
METHODS: gp96 isolated from livers of normal mice and its N-terminal fragment (amino acid 22-355) expressed in E coli were used for immunization of BALb/c mice. Eight groups of mice received one of the following regiments subcutaneously in 100 μL phosphate buffered saline (PBS) at an interval of 3 wk. Group 1: PBS only; group 2: gp96 only; group 3: N-terminal fragment only; group 4: HBsAg only; group 5: HBsAg+gp96; group 6: HBsAg+N-terminal fragment; group 7: HBsAg+incomplete Freud’s adjuvant; group 8: HBsAg+N-terminal fragment (95 °C heated for 30 min). Serum anti-HBsAg antibody levels were assayed by ELISA. CTL responses in splenocytes were analyzed by ELISPOT after the last vaccination.
RESULTS: The average titer of serum anti-HBsAg antibody in the mice immunized with HBsAg together with gp96 or its N-terminal fragment were much higher than those immunized with HBsAg alone detected by ELISA. The cellular immune response of the mice immunized with HBsAg together with gp96 or its N-terminal fragment was not different with those immunized with HBsAg alone measured by ELISPOT assay.
CONCLUSION: gp96 or its N-terminal fragment greatly improved humoral immune response induced by HBsAg, but failed to enhance the CTL response, which demonstrated the potential of using gp96 or its N-terminal fragment as a possible adjuvant to augment humoral immune response against HBV infection.
Keywords: Heat shock protein, gp96 N-terminal fragment, HBV, Hepatitis B virus surface antigen (HBsAg), Vaccine
INTRODUCTION
Heat shock protein gp96 (HSP gp96; glucose-regulated protein, GRP94), as a member of HSP90 family, is one of the most abundant proteins in cells, which displays various roles besides protein folding and assembly[1,2]. But most attractive feature of gp96 is its contributions to immune system[3]. The phenomena that gp96 isolated from tumors or virus-infected cells elicit specific CTLs against their origins are widely observed[4-10]. It is believed that gp96 is capable of channeling antigenic peptides to MHC class I presentation pathway of antigen presenting cell (APC) by acting as ligand of CD91[11-14]. The immunologic processes activated in response to tumor antigen negative sources of GRP94/gp96 are currently presumed to be associated with the stimulation of CD11b (+) and CD11c (+) APCs and activation of the bystander CD4 (+) T cell Th1 cytokine production[15]. Autologous vaccines based on general properties of gp96 have been widely studied, but there exists obvious limitations including quantity of gp96 for therapy, which are strictly limited by the size of the resected tumor mass[16]. Moreover, only half of the treated patients vaccinated with gp96 derived from autologous tumor induced an antitumor response in phase I/II clinical trials[17]. Hence, a novel HSP-mediated universally applicable vaccine is still in need. Ideal therapeutic vaccines for infectious diseases and cancer might elicit not only the cellular response but also the humoral response. However, the studies so far on the immune effect of gp96 were mainly focused on its enhancement of CTLs, hereby we wanted to check whether the gp96 derived from normal tissue could influence humoral immune response and act as a regular adjuvant. However, it was hard for such a big molecule expressed in E.coli by our experimental practice, so gp96 purified from normal tissue was used as the source of the protein.
The N-terminal domain (amino acids 1-263), homologous to the proteolytic fragment of HSP90, was regarded as the nucleotide-binding domain[18], followed by acidic residues (amino acids 264-344). The first 355 amino acids of GRP94 were found to be peptide-binding sites, capable of binding a number of peptides[19,20]. Furthermore, it is reported that the N-terminal peptide binding domain fragment could suppress tumor growth like that of the full gp96[21]. In order to check the idea of using recombinant N-terminal fragment as analog of gp96, and investigate its immune effect, we expressed the N-terminal fragment of gp96 in pGEX-6p1 vector in E.coli. After co-immunization with gp96 or the N-terminal fragment and HBsAg, the humoral and cellular immune responses in mice were compared with those of administration HBsAg alone. Some concern that the immune responses induced by recombinant HSPs produced in E.coli might result from the effects of lipopolysaccharide (LPS) contaminated during the preparation[22]. Therefore, necessary measures were taken to avoid the possible influence by endotoxin.
MATERIALS AND METHODS
Recombinant HBsAg
Nonglycosylated HBsAg, subtypes adw, containing the small HBsAg (S) protein of HBV derived from the Saccharomyce cerevisiae host strain 2150-2-3 carrying pHBS56-GAP347/33 plasmid containing S-gene encoding HBsAg was obtained from Dr. Chin-Yuan Guo (Beijing Tiantan Biological Products Co., Ltd). Briefly, the harvested fermentation product of the yeast was homogenized to release HBsAg, the ruptured yeast cells were microfiltered and ultrafiltered to remove large debris and small molecule contaminants, the HBsAg particles was further purified by silica adsorption and butyl-agarose hydrophobic interaction chromatography consequently. The purity and pyrogen were tested by high performance size exclusion liquid chromatography on TSK G4000SW column and Limulus Amebocyte Lysate for endotoxin detection reagent (Chinese Horseshoe Crab Reagent Manufactory), which are higher than 99.0% and lower than 10 EU/mL respectively.
gp96 expressed in E coli and purification of gp96 from normal liver tissue
The gene encoding murine gp96 was donated by Professor Srivastava (GenBank Accession No. gi: 6755862). The gene encoding amino acids 22-802 of gp96 was amplified by PCR and cloned into E.coli expression vector pET30a (Novagen) by two restriction enzyme sites of BamHI and XhoI. A stopcodon was introduced immediately before the XhoI site. This cloning strategy yielded fusion proteins, in which there are extra 50 amino acids in total at the N-terminus of gp96. The recombinant plasmids of pET30a-gp96 were transformed into E.coli strain BL21 (DE3). Bacterial cells were harvested and lysed by sonication in phosphate buffered saline (PBS). The clarified supernatants were applied on Ni-chelated Sepharose affinity column (Pharmacia). The column then was washed by PBS and eluted with imidazole (100 mmol/L). The eluted material was applied to POROS 20QE column (4.6-100 mm, PE Biosystem, Foster City, CA, USA) on an AKTA fast protein liquid chromatography (FPLC) Workstation with a 300-800 mmol/L NaCl gradient. Fractions (1 mL) were collected and analyzed by 10% SDS-PAGE.
Native gp96 was purified as described previously[23]. Healthy mouse liver tissues were suspended in 30 mmol/L sodium bicarbonate. The lysate was centrifuged to remove nuclei and other debris. The supernatant was precipitated with ammonium sulfate (50-70%). The solubilized precipitation was applied to a ConA-Sepharose column (Pharmacia Biotech, Uppsala, Sweden). The column was eluted with buffer containing 10% a-methylmanno pyranoside (Sigma). The eluted material was applied to POROS 20QE column on a AKTA FPLC Workstation with a 300-800 mmol/L NaCl gradient. Fractions (1 mL) were collected and analyzed by 10% SDS-PAGE. The resultant protein was applied to a Gel filtration Superdex G200 on a AKTA FPLC Workstation. The identity of the gp96 protein was confirmed by Western blot using anti-gp96 monoclonal antibody (NeoMarkers, Fremont, USA).
Expression and purification of the N-terminal fragment of gp96
The N-terminal fragment (NTF) of gp96 (from 22aa to 355aa) was cloned into the BamHI and XhoI sites of GST fusion expression vector pGEX-6p-1 (Pharmacia), containing PreScission protease cleavage site (Sigma). The positive plasmids were verified by direct DNA sequencing. E.coli strain BL21 (DE3) transformed with the recombinant pGEX-6p-1 plasmid was grown at 37 °C in 2×YTA medium before induction with 1 mmol/L IPTG for 4 h at 37 °C. Bacterial cells were harvested and lysed by sonication in PBS. The clarified supernatants of the lysate were passed through a glutathione±Sepharose 4B column. The GST fusion protein-bound column was washed with PBS and eluted with reduced glutathione. After cleavage the free GST and the protease were removed by passage through the glutathione±Sepharose 4B column again. The resultant protein was applied to a Gel filtration Superdex G75 on AKTA FPLC Workstation. SDS-PAGE and Western blot were used to identify the protein. The quantities of proteins were estimated by protein concentration test kit (BioRad). Additionally, all material for protein preparations were treated prior to use, to remove possible contaminants of endotoxin. All buffers were made in pyrogen-free water.
Immunization in mice
Female Balb/c mice were obtained from the Animal Research Center of Medical Department in Peking University (Beijing, China). Eight groups of mice were immunized with one of the following regiments in 100 μL PBS : (1) PBS; (2) 10 μg NTF only; (3) 10 μg gp96 only; (4) HBsAg alone; (5) mixture of 10 μg NTF and 10 μg HBsAg; (6) mixture of 10 μg gp96 and 10 μg HBsAg; (7) 10 μg of HBsAg emulsified in incomplete Freud’s adjuvant (IFA); (8) mixture of 10 μg NTF (heated at 95 °C for 30 min) and 10 μg HBsAg. All groups contained at least 8 mice. All injections were done subcutaneously at 0, 3 and 6 wk.
HBsAg-specific antibody assay
Sera samples were collected from all mice by tail bleeding at different times and the presence of HBsAg-specific antibody was analyzed by ELISA. The ELISA kits for the HBsAg-specific antibody detection were purchased from Sino-American BioTechnology Co., China and operated following the manufacturer’s instructions 2 wk after the last immunization to compare the A values from different groups. HBsAg-specific IgG serum Abs were determined by an end-point dilution ELISA assay. Micro-ELISA plates (costar; Corning Incorporated, NY, USA) were coated with 150 ng recombinant HBsAg particles per well in 50 µL 0.1 mol/L sodium carbonate buffer (pH 9.5) at 4 °C. Serial dilutions of the sera in loading buffer (PBS supplemented with 3% BSA and 2% Tween 20) were added to the antigen-coated wells. Serum Abs were incubated for 2 h at 37 °C before four washes with PBS supplemented with 0.05% Tween 20. Bound serum Abs were detected using HRP-conjugated goat anti-mouse IgG Ab (catalog no. 214-1 806; KPL, USA) before incubation with substrate o-phenylenediamine (Sigma) in PBS. The reaction was stopped by 1 M H2SO4, and determined at 450 nm. End-point titers were defined as the highest serum dilution that resulted in an absorbance value three times greater than that of negative control sera (derived from PBS immunized BALb/c mice).
CTL assay
Spleen cells of mice were segregated 2 wk after the last immunization and the CTL activities were measured by ELISPOT assay, which was performed according to the instruction of the murine IFN-γ ELISPOT kit (Diaclone, France). Briefly, 96-well polyvinylidene difluoride-backed plates were precoated with 15 µg/mL anti-IFN-γ mAb overnight at 4 °C. Plates were blocked for 1 h at 37 °C. Purified splenocytes were dispensed at predetermined density in duplicate wells. Ten micromole per liter of peptide (WYWGPSLYSI, GL Biochem, Shanghai) was used to stimulate the cells[24]. The plates were incubated at 37 °C for 18-40 h. After washing, the plates were then incubated for another 1.5 h at 37 °C after addition of the biotinylated anti-IFN-γ antibody. A 1:1000 dilution of streptavidin-alkaline phosphatase conjugate was then added into the wells and incubated for 1 h after which the chromogenic alkaline phosphatase substrate was added. After 30 min, the colorimetric reaction was terminated by washing with tap water. After drying, the spots were counted.
Statistical analysis
The statistical significance of the difference between two groups were determined by the two-tailed Student’s t test and was set to P<0.05.
RESULTS
Expression and purification of gp96
gp96 were expressed as fusion protein with an extra 50 amino acids at their N-terminus derived from the expression vector pET30a, including the 6× his-tag and two protease cleavage sites (thrombin and enterokinase). The expressed proteins were purified with Ni-chelated affinity column. In the step of the purification on the POROS 20QE column, gp96 was eluted within a wide range of salt concentration (400-700 mmol/L NaCl). The product contained mostly degraded fragments of gp96 or its aggregations identified by 10%SDS-PAGE (Figure 1A). The purity of resultant proteins could not meet the demand of immunization. So we resorted to purify the gp96 from the normal tissue.
Figure 1.

Gp96 expressed in E.coli and purification from normal murine livers. A and B: Elutions of gp96 from POROS 20QE column with 300-800 mmol/L NaCl were detected by SDS-PAGE. The gp96 expressed in E.coli. A: or purified from mice livers; B: were collected as 1 mL fractions during the purification on POROS 20QE column and a total of six individual fractions (lanes 1-6) were run on the SDS-PAGE gel with Coomassie blue staining afterwards; C: Gel-filtration analysis of gp96 purified from mice livers. The protein samples are run on Superdex G200 column; D: Proteins collected from the peak of gel filtration were run on 10% SDS-PAGE and Western blot are shown on the right.
Approximately 20-30 mg of apparently homogeneous preparations of gp96 were obtained from each gram of wet weight of the healthy murine lives by our method[23]. Unlike the recombinant gp96, all peak fractions in the wide range contained apparently homogeneous gp96. The purity of gp96 preparations were determined by 10% SDS-PAGE (Figure 1B). Fractions containing gp96 were pooled and further purified on a gel-filtration Superdex G200 column (Figure 1C). Using the purification procedures mentioned above, we routinely obtained gp96 of over 95% purity as judged by SDS-PAGE and Western blot (Figure 1D).
N-terminal fragment of gp96 was expressed as soluble proteins
E.coli is widely useful to produce target proteins for its high production and convenient manipulation, but the full-length gp96 molecule may be too big to produce in BL21 cells. Although the gp96 N-terminal fragment could stimulate maturation of APC and suppress tumor growth was reported, whether the fragment can generate immune effects is unknown[21]. In order to investigate the idea of using recombinant N-terminal fragment as analog of gp96 and address its immune effect, the N-terminal fragment of gp96, NTF (22-355 aa) was cloned into pGEX-6p1 expression vector, mainly expressed as fusion proteins to GST in supernatant of bacteria BL21 cells lysate (Figure 2A). The gel-filtration Superdex G75 column was applied for further purification after removing GST (Figure 2B). The resultant proteins were analyzed by Coomassie blue staining to be at least 95% pure (Figure 2C) and immunoblotted with an anti-gp96 monoclonal antibody (Figure 2C). NTF was recognized by the monoclonal antibody consistent with the pervious report[25]. Comparing with the recombinant gp96, NTF was more stable and purer.
Figure 2.
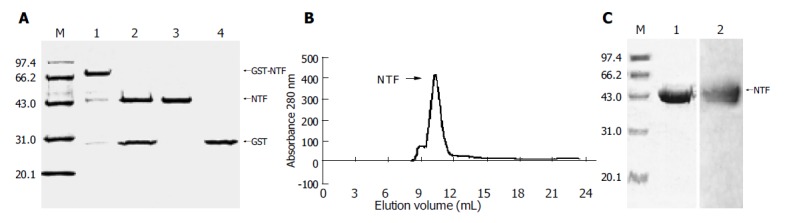
Expression and purification of the N-terminal fragment of gp96 in the GST fusion expression system. A: The resulting protein N-terminal fragment of gp96 (named after NTF) was separated from the digestion product on a glutathione±Sepharose 4B column to remove GST and GST-3C protease. Lane 1, 10% SDS±PAGE of GST-NTF eluted by reduced glutathione; lane 2, NTF after GST-3C protease digestion (16 h, 5 °C); lane 3, NTF after removing GST and GST-3C protease; lane 4, purified GST as control; M, protein molecular weight markers as indicated in kilo Daltons; B: Gel-filtration analysis of NTF protein. NTF protein subjected to Superdex G75 gel-filtration; C: The proteins from the peak run on 10% SDS-PAGE and Western blot. The standard proteins are superimposed in both A and C.
Gp96 and its N-terminal fragment can enhance the humoral response to HBsAg
The A values of anti-HBsAg antibody from different groups were compared at day 63 after three doses of immunization (Figure 3A). Mice were immunized with HBsAg emulsified in IFA and PBS as positive and negative control respectively (Figure 1A: groups 1 and 7). NTF alone or gp96 alone had no effect on the antibody production (Figure 1A: groups 2 and 3). A strong anti-HBsAg response was observed in mice after three doses of immunization with the mixture of HBsAg and NTF, gp96 or IFA (groups 5, 6 and 7). The highest A value was from the mice immunized with HBsAg and NTF (group 5). The values of antibody were not significantly different between the groups immunized with HBsAg plus NTF (group 5) or gp96 (group 6) compared to those of HBsAg plus IFA (group 7) (P>0.05). However, the values of anti-HBsAg were significantly correlated with the mice immunized with or without the assistance of adjuvant to HBsAg (P<0.05).
Figure 3.
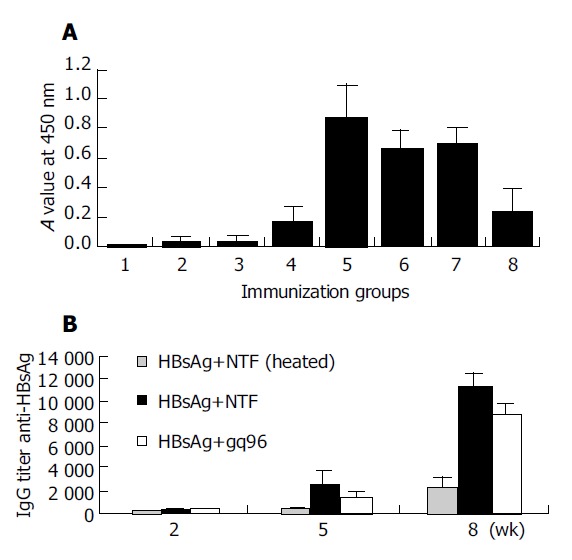
Induced humoral immune response in Balb/c mice. A: Groups (eight mice per group) of Balb/c mice were immunized with different gradient: group 1, PBS only; group 2, 10 μg gp96 only; group 3, 10 μg NTF only; group 4, 10 μg HBsAg only; group 5, 10 μg HBsAg+10 μg NTF; group 6, 10 μg HBsAg+10 μg gp96; group 7, 10 μg HBsAg+IFA; group 8, 10 μg HBsAg+10 μg NTF (95 °C heated for 30 min) at d 0 (day of first vaccination). At d 21, and 42 mice were re-immunized with the same material. At d 63 anti-HBsAg antibodies from different groups were compared using the value at 450 nm absorbance. The values of antibody were expressed as geometry mean±SD; B: Sera from the mice immunized with HBsAg plus heated NTF, HBsAg plus NTF or gp96 (groups 5, 6 and 8) were collected on d 14, 35 and 64 from first vaccination and stored at -20 °C and titers at different dilution were tested at the same time.
In order to investigate whether the levels of anti-HBsAg were correlated with the time after immunization, and to examine the detailed difference between the mice immunized with HBsAg and those immunized with HBsAg and NTF or gp96, the end-point dilution was used to compare the anti-HBsAg IgG 2 wk after each vaccination (Figure 3B). There was no significant difference in anti-HBsAg antibody level between groups immunized with HBsAg alone and those immunized with HBsAg plus heated NTF, which demonstrated that the LPS contamination had no influence on the results (Figure 3A: groups 4 and 8). Therefore, we added the mice immunized with HBsAg plus heated NTF to the group of mice immunized with HBsAg alone in the following tests. Significant difference was observed between the mice immunized with HBsAg plus heated NTF and those immunized with HBsAg plus NTF or gp96 after two doses of immunization (Figure 3B) (P<0.05). The highest end-point dilution titers were achieved in mice after three doses of immunization with HBsAg plus NTF or gp96 (Figure 3B), which were 5-10 fold higher than those with HBsAg plus heated NTF. The above results showed that gp96 and its N-terminal fragment enhanced the humoral response to HBsAg.
Gp96 and its N-terminal fragment failed to enhance the CTL response to HBsAg
Whether the CTLs could be obtained when the two proteins were simply mixed but not covalently bonded[26] were examined. The number of spot forming cells (SFCs) representing the IFN-γ secreted CTLs, in splenocytes in response to the HBsAg epitope WYWGPSLYSI in vitro was measured by ELISPOT. Splenocytes stimulated with ConA served as the positive control. Splenocytes from mice immunized with HBsAg with gp96 or a mixture of HBsAg and NTF produced the same level of SFCs in response to stimulation with the peptide (Figure 4). While there were differences between the mice immunized with PBS and those with HBsAg plus adjuvant, there were no significant differences between mice immunized with HBsAg plus heated NTF and those with HBsAg plus gp96 or NTF (Figure 4). It is conceivable that this lack of immune enhancement effect was likely due to inability for gp96 to chaperone large exogenous antigen into MHC class I pathway of APC.
Figure 4.
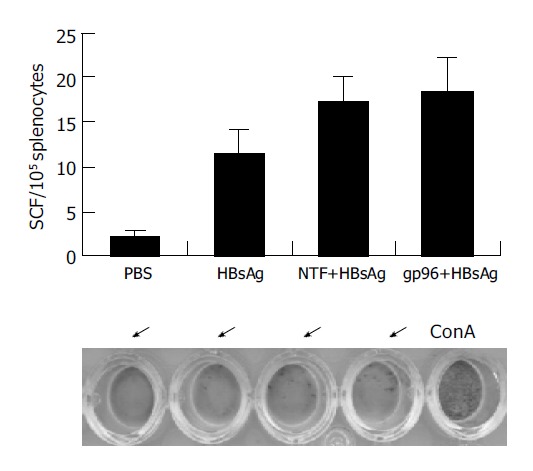
T-cell response of splenocytes of immunized Balb/c mice. Mice immunized with PBS, HBsAg plus heated NTF, HBsAg plus NTF or gp96 (the same groups as groups 5, 6, and 8 respectively in Figure 3) were killed on d 63 and the splenocytes were collected for ELISPOT assay with 105 cells per well. The number of SFCs was calculated. The photograph of the representative wells from different groups is shown below the statistics.
DISCUSSION
During the process of studies on tumor transplantation and rejection, two important groups of proteins were found: MHC proteins and HSPs. HSPs appeared earlier in evolution, more conservative in structure and more abundant in the cells[27]. As a member of HSPs, gp96 has similar biological characteristics to protein chaperones. The most attractive function of gp96 with respect to medicinal applications is its immune role upon both the innate and adaptive immune systems[28]. Previously the main interests towards gp96 were the focus on its peptide chaperone and its involvement in the pathway of MHC class I to enhance CTL responses. Recently reports have disclosed the effect of gp96 upon the pathway of MHC class II[29-31]. Our results illustrate for the first time its enhancement of the humoral response to protein antigen HBsAg, which provides evidence that gp96 can influence the antibody production. The reason that gp96 is enhancing humoral response might be due to (1) stimulation of APC to increase the efficiency of processing exogenous antigen and presenting peptides to MHC class II molecules; (2) activation of bystander CD4+ T cells; (3) functions directly as a Th2-specific costimulatory molecule. A protein vaccine can potentially boost immune response, but without suitable adjuvant, soluble proteins can only elicit a weak immune response[32,33]. Gp96 could act as a potent adjuvant to increase humoral responses by this study. Whether the covalently linked gp96 and HBsAg can elevate both the antibody and CTL specific to HBsAg needs further investigation.
In the world, the number of individuals infected by HBV is over 400 million. About 60% of the world’s 530000 cases of liver cancer per year are caused by viral hepatitis B infection[34]. Interferon and nucleoside analogs are still the most effective drugs to treat chronic hepatitis B. However, cessation of treatment usually leads to a rapid relapse of disease, and long-term treatment often results in the selection of drugs resistant viral variants[35]. Therapeutic vaccines have been proposed to break the established T-cell tolerance in chronically infected patients[36,37]. Recently it was suggested that continuous stimulation of dendritic cells (DCs) by ligands of Toll-like receptors was needed to overcome CD4+CD25+ T cell-mediated suppression and mature DCs alone were not sufficient to break CD8 tolerance[38]. Immunotherapeutic vaccines based on gp96 conceived in our lab maybe an optimal candidate to break the established T-cell tolerance because gp96 can act as ligand of pattern recognition receptors[39]. However, the whole-length of gp96 was poorly expressed in E.coli and the product after purification was produced in unacceptably low amounts and was unstable, which limited its applications. Our results showed that the N-terminal fragment of gp96 expressed very well in E.coli with more production and higher quality, and owned the same effect as the full-length gp96 to increase the antibody to HBsAg. Further studies on the N-terminal fragment of gp96 and the HBV infection will bring us more knowledge for better usage of this fragment to fight against HBV infection.
ACKNOWLEDGMENTS
We are grateful to Dr. Gordon Laity for helping in the preparation of this manuscript, Dr. Song-Dong Meng for critical reading of the manuscript, Fu-Lian Liao for technical assistance, Professor Pramod K. Srivastava for providing the mouse gp96 clone, Professor Wei-Feng Chen of Peking University and Professor Xue-Tao Cao of the Second Military Medical University for help in various stages of the project, and Wei-Hua Zhuang for graphic preparation.
Footnotes
Supported by the Major State Basic Research Development Program of China, Program 973, Grant No. 2001CB510001
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 2.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 4.Rivoltini L, Castelli C, Carrabba M, Mazzaferro V, Pilla L, Huber V, Coppa J, Gallino G, Scheibenbogen C, Squarcina P, et al. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J Immunol. 2003;171:3467–3474. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165–171. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- 6.Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother. 2003;52:226–234. doi: 10.1007/s00262-002-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchianò A, Andreola S, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–3245. [PubMed] [Google Scholar]
- 8.Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–238. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 10.Manjili MH, Wang XY, Park J, Facciponte JG, Repasky EA, Subjeck JR. Immunotherapy of cancer using heat shock proteins. Front Biosci. 2002;7:d43–d52. doi: 10.2741/manjili. [DOI] [PubMed] [Google Scholar]
- 11.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 12.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 13.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci USA. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng SD, Gao T, Gao GF, Tien P. HBV-specific peptide associated with heat-shock protein gp96. Lancet. 2001;357:528–529. doi: 10.1016/S0140-6736(00)04050-2. [DOI] [PubMed] [Google Scholar]
- 15.Baker-LePain JC, Sarzotti M, Nicchitta CV. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J Immunol. 2004;172:4195–4203. doi: 10.4049/jimmunol.172.7.4195. [DOI] [PubMed] [Google Scholar]
- 16.Gordon NF, Clark BL. The challenges of bringing autologous HSP-based vaccines to commercial reality. Methods. 2004;32:63–69. doi: 10.1016/s1046-2023(03)00188-9. [DOI] [PubMed] [Google Scholar]
- 17.Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, Mazzaferro V, Coppa J, Srivastava PK, Parmiani G. Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 2004;53:227–233. doi: 10.1007/s00262-003-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 19.Vogen S, Gidalevitz T, Biswas C, Simen BB, Stein E, Gulmen F, Argon Y. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J Biol Chem. 2002;277:40742–40750. doi: 10.1074/jbc.M205323200. [DOI] [PubMed] [Google Scholar]
- 20.Gidalevitz T, Biswas C, Ding H, Schneidman-Duhovny D, Wolfson HJ, Stevens F, Radford S, Argon Y. Identification of the N-terminal peptide binding site of glucose-regulated protein 94. J Biol Chem. 2004;279:16543–16552. doi: 10.1074/jbc.M313060200. [DOI] [PubMed] [Google Scholar]
- 21.Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–1459. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed RC, Berwin B, Baker JP, Nicchitta CV. GRP94/gp96 elicits ERK activation in murine macrophages. A role for endotoxin contamination in NF-kappa B activation and nitric oxide production. J Biol Chem. 2003;278:31853–31860. doi: 10.1074/jbc.M305480200. [DOI] [PubMed] [Google Scholar]
- 23.Meng SD, Song J, Rao Z, Tien P, Gao GF. Three-step purification of gp96 from human liver tumor tissues suitable for isolation of gp96-bound peptides. J Immunol Methods. 2002;264:29–35. doi: 10.1016/s0022-1759(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 24.Falk K, Rötzschke O, Stevanović S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 25.Sargan DR, Tsai MJ, O'Malley BW. hsp108,,,,,, a novel heat shock inducible protein of chicken. Biochemistry. 1986;25:6252–6258. doi: 10.1021/bi00368a062. [DOI] [PubMed] [Google Scholar]
- 26.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 29.Baker-LePain JC, Sarzotti M, Nicchitta CV. Glucose-regulated protein 94/glycoprotein 96 elicits bystander activation of CD4+ T cell Th1 cytokine production in vivo. J Immunol. 2004;172:4195–4203. doi: 10.4049/jimmunol.172.7.4195. [DOI] [PubMed] [Google Scholar]
- 30.Doody AD, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee PP, Vinay DS, Mathew A, Raje M, Parekh V, Prasad DV, Kumar A, Mitra D, Mishra GC. Evidence that glycoprotein 96 (B2), a stress protein, functions as a Th2-specific costimulatory molecule. J Immunol. 2002;169:3507–3518. doi: 10.4049/jimmunol.169.7.3507. [DOI] [PubMed] [Google Scholar]
- 32.Hansson M, Nygren PA, Ståhl S. Design and production of recombinant subunit vaccines. Biotechnol Appl Biochem. 2000;32(Pt 2):95–107. doi: 10.1042/ba20000034. [DOI] [PubMed] [Google Scholar]
- 33.Liljeqvist S, Ståhl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J Biotechnol. 1999;73:1–33. doi: 10.1016/s0168-1656(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 34.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 35.Liaw YF. Therapy of chronic hepatitis B: current challenges and opportunities. J Viral Hepat. 2002;9:393–399. doi: 10.1046/j.1365-2893.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- 36.Soemohardjo S. New options in the treatment of chronic hepatitis. Adv Exp Med Biol. 2003;531:191–198. doi: 10.1007/978-1-4615-0059-9_15. [DOI] [PubMed] [Google Scholar]
- 37.Pol S, Driss F, Michel ML, Nalpas B, Berthelot P, Brechot C. Specific vaccine therapy in chronic hepatitis B infection. Lancet. 1994;344:342. doi: 10.1016/s0140-6736(94)91384-6. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 39.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]


