Abstract
AIM: To isolate nestin-positive progenitor cells from human fetal pancreas and to detect their surface markers and their capability of proliferation and differentiation into pancreatic islet endocrine cells in vitro.
METHODS: Islet-like cell clusters (ICCs) were isolated from human fetal pancreas by using collagenase digestion. The free-floating ICCs were handpicked and cultured in a new dish. After the ICCs developed into monolayer epithelium-like cells, they were passaged and induced for differentiation. Reverse transcription polymerase chain reaction (RT-PCR), immunofluorescence stain, fluorescence-activated cell sorting (FACS) and radioimmunoassay (RIA) were used to detect the expression of cell markers.
RESULTS: (1) The monolayer epithelium-like cells had highly proliferative potential and could be passaged more than 16 times in vitro; (2) RT-PCR analysis and immunofluorescence stain showed that these cells expressed both nestin and ABCG2, two of stem cell markers; (3) FACS analysis revealed that CD44, CD90 and CD147 were positive, whereas CD34, CD38, CD45, CD71, CD117, CD133 and HLA-DR were negative on the nestin-positive cells; (4) RT-PCR analysis showed that the mRNA expression of insulin, glucagon and pancreatic-duodenal homeobox gene-1 was detected, whereas the expression of nestin and neurogenin 3 disappeared in these cells treated with serum-free media supplemented with the cocktail of growth factors. Furthermore, the intra-cellular insulin content was detected by RIA after the induction culture.
CONCLUSION: Nestin-positive cells isolated from human fetal pancreas possess the characteristics of pancreatic progenitor cells since they have highly proliferative potential and the capability of differentiation into insulin-producing cells in vitro. Interestingly, the nestin-positive pancreatic progenitor cells share many phenotypic markers with mesenchymal stem cells derived from bone marrow.
Keywords: Fetus, Nestin, Pancreas, Stem cells
INTRODUCTION
Pancreatic β-cell replacement therapy via islet transplantation has become a subject of intense interest again, because the recent success rate of the procedure is largely improved by using the Edmonton protocol. However, this successful new protocol utilizes the islets isolated from at least two human cadaveric pancreata[1]. In order to make such a therapy available to more than a few of thousands of patients with diabetes, new sources of insulin-producing cells must be identified. Pancreatic stem cells have the capacity to expand and differentiate into pancreatic islet cells, and are therefore considered as the potential donor source for islet transplantation.
A definitive molecular marker of pancreatic stem cells remains obscure. One recent study demonstrated that nestin-positive cells resided in adult human and rat islets had the characteristics of stem cells and could be differentiated into pancreatic endocrine, exocrine and hepatic cell phenotypes in vitro[2]. Therefore, it has been firstly proposed that nestin is a maker for pancreatic stem cells. Another more recent report showed that nestin-positive precursors derived from human fetal pancreas could differentiate into pancreatic endocrine cells, which displayed the ability to reverse hyperglycemia in diabetic mice[3]. On the other hand, several other publications have recently suggested that nestin-positive cells in either adult or fetal pancreata were unable to generate pancreatic islet β-cells in vitro[4-6]. This controversy may reflect that nestin-positive pancreatic cells may be a heterogeneous cell population, or the protocol for inducing their differentiation must be optimized.
In this study, nestin-positive cells were isolated from human fetal pancreata, and then reverse transcription polymerase chain reaction (RT-PCR), immunofluorescence stain, fluorescence-activated cell sorting (FACS) and radioimmunoassay (RIA) were used to determine whether the nestin-positive pancreatic cells had the characteristics of stem cells.
MATERIALS AND METHODS
Isolation, culture and induction of pancreatic progenitor cells
Human fetal pancreata at 20th gestational weeks were provided by Department of Obstetrics and Gynecology, Peking University First Hospital after the termination of pregnancy. Informed consent for tissue donation was obtained by the procurement centers. In addition, our institutional review board had reviewed and approved the use of fetal tissue for these studies. The pancreata were received within 8 h after procurement and enzymatically digested as previously described[7]. The digested tissue was washed twice in cold HBSS and placed on 100-mm bacteria Petri dishes in RPMI 1640 media (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone), 10 mmol/L HEPES, 1 mmol/L sodium pyruvate and 71.5 µmol/L β-mercaptoethanol (Merck, Darmstadt, Germany). After incubation for 96 h, the floating islet-like cell clusters (ICCs) were pipetted out and cultured in new dishes with fresh media supplemented with 20 ng/mL basic fibroblast growth factor and 20 ng/mL epidermal growth factor (both from Invitrogen, Carlsbad, CA, USA). The ICCs attached to the bottom and a monolayer of epithelium-like cells started the outgrowth from the ICCs within 24 h. After confluence, the cells were repeatedly passaged. After about 80% confluence in the cells passaged 10 times, a serum-free medium supplemented with 100 pmol/L hepatocyte growth factor (Chemicon, Temecula, CA, USA), 2 nmol/L activin A (R&D Systems, Minneapolis, MN, USA), 500 pmol/L betacellulin, 10 nmol/L exendin-4 (glucagon-like peptide-1 analog) and 10 mmol/L nicotinamide (all three from Sigma, St. Louis, MO, USA) in a low-glucose concentration (5 mmol/L) was changed, and the cells were further cultured for another 6 d.
Immunofluorescence
The expanded pancreatic progenitor cells were grown on coverslips and processed as previously described[8]. Briefly, the cells were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature. The cells were rinsed with PBST (PBS with 0.5% Triton X-100) thrice and incubated in PBS containing 10% goat serum and 0.01% Triton X-100 for blocking, and then incubated overnight at 4 °C with mouse anti-human nestin monoclonal antibody (1:100; BD Transduction Laboratories, Lexington, KY, USA). After washing thrice with PBST, the cells were incubated with FITC-conjugated goat anti-mouse IgG (1:100; BD Transduction Laboratories) for 1 h at room temperature. Following three washes with PBS, the samples were mounted on slides and examined with an Olympus BX51 fluorescence microscope (Olympus Optical, Tokyo, Japan) equipped with a Micro Color RGB-MS-C CCD camera (CRI, Woburn, MA, USA).
Identification of cell surface markers by FACS
2×105 of the cells passaged 10 times were resuspended in 100 µL of PBS and incubated with FITC-conjugated CD34, CD38, CD45, CD90 and HLA-DR antibodies, and PE-conjugated CD44, CD71, CD117, CD133 and CD147 antibodies (all from BD PharMingen, San Diego, CA, USA) for 30 min at 4 °C. After washing with PBS twice, the labeled cells were analyzed in a flow cytometer (Becton-Dickinson, San Jose, CA, USA).
RNA extraction and RT-PCR analysis
RNA was isolated from the cultured pancreatic progenitor cells using TRIzol (Gibco, Carlsbad, CA, USA) following the manufacturer’s protocol. Single-stranded cDNA was prepared with the Superscript First-Strand System (Invitrogen) as described previously[9]. The cDNAs were amplified by polymerase chain reaction (PCR). The PCR products were analyzed by 1% agarose gel electrophoresis. G3PDH, nestin, ABCG2, pancreatic-duodenal homeobox gene-1 (PDX-1), insulin, and glucagon were amplified for 30, 40, 34, 35, 36 and 23 cycles, and the annealing temperatures were 50, 55, 56, 52, 62, and 59 °C, respectively. The annealing temperature of neurogenin 3 (Ngn3) was descended from 68 to 57 °C at 1 °C every cycle, and then the PCR reaction was run for 29 cycles. The sequences of primers were as follows: 5’ ACC ACA GTC CAT GCC ATC AC 3’ and 5’ ATG TCG TTG TCC CAC CAC CT 3’ for G3PDH; 5’ AGC GTT GGA ACA GAG GTT GGA 3’ and 5’ TGT TTC CTC CCA CCC TGT GTC T 3’ for nestin; 5’ GGC CTC AGG AAG ACT TAT GT 3’ and 5’ AAG GAG GTG GTG TAG CTG AT 3’ for ABCG2; 5’ AGA GCG AGT TGG CAC TGA G 3’ and 5’ CTG AGA AAG CCA GAC TGC C 3’ for Ngn3; 5’ CCC ATG GAT GAA GTC TAC C 3’ and 5’ GTC CTC CTC CTT TTT CCA C 3’ for PDX-1; 5’ GCC TTT GTG AAC CAA CAC CTG 3’ and 5’ GTT GCA GTA GTT CTC CAG CTG 3’ for insulin; and 5’ ATG AAC GAG GAC AAG CGC 3’ and 5’ TTC ACC AGC CAA GCA ATG 3’ for glucagon.
Measurement of intracellular insulin content by RIA
For the determination of insulin content, insulin was extracted from the cells before and after induction following standard protocol[10]. The cells were washed twice in PBS, and treated with acid ethanol (10% glacial acetic acid in absolute ethanol) overnight at 4 °C, followed by cell sonication. Total protein in the lysates was determined by Biophotometer (Eppendorf, Hamburg, Germany). Insulin was measured by using a solid-phase radioimmunoassay (RIA) kit (DPC, Los Angeles, CA, USA).
RESULTS
Nestin-positive progenitor cells isolated from human fetal pancreas
After a 96-h incubation, the free-floating ICCs were handpicked and placed into a new dish. Within 24 h, the ICCs attached to the bottom and epithelium-like cells spread out from the ICCs (Figures 1A-1C). The cells were able to be passaged more than 16 times in vitro. To confirm the existence of nestin-positive cells, an immunofluorescence study was conducted in the cultured pancreatic cells. Nestin expression was detected in the monolayer cells that grew out from the ICCs and in those that passaged for 10 times (Figures 1D-1F). RT-PCR showed that both nestin and ABCG2 were expressed in the passaged cells (Figure 2).
Figure 1.
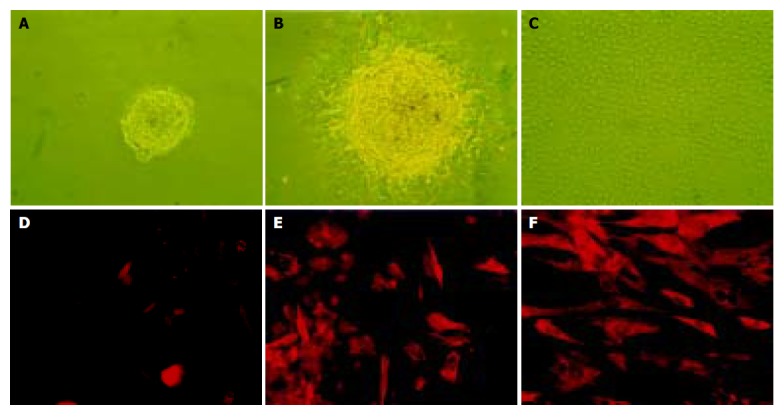
The culture (A-C) and immunofluorescence staining (D-F) of nestin-positive cells isolated from human fetal pancreas. A: The free-floating ICCs isolated from fetal human pancreas; B: After pipetted out and cultured in a new dish, the ICCs attached to the bottom and a monolayer of epithelium-like cells spread out; C: The epithelium-like cells making confluent sheet, the ICCs disappeared structurally (×100); D: Negative control using mouse IgG to substitute the primary antibody; E: Nestin expressed in some of the epithelium-like cells spreading out from ICCs. F: Expression of nestin in most of the epithelium-like cells passaged 10 times (×200).
Figure 2.
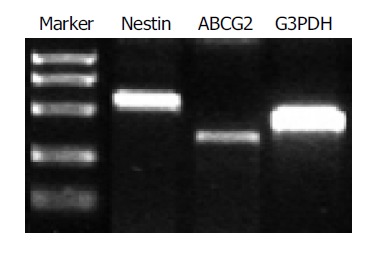
RT-PCR analysis of nestin (549 bp) and ABCG2 (342 bp) expression in the cultured cells. G3PDH (452 bp) served as internal control.
Surface markers of the nestin-positive pancreatic progenitor cells
FACS analysis showed that CD44, CD90 and CD147 were positive, whereas CD34, CD38, CD45, CD71, CD 117, CD133 and HLA-DR were negative in the nestin-positive pancreatic progenitor cells (Figure 3), suggesting that the phenotype of these cells was identical to that of mesenchymal stem cells derived from bone marrow.
Figure 3.
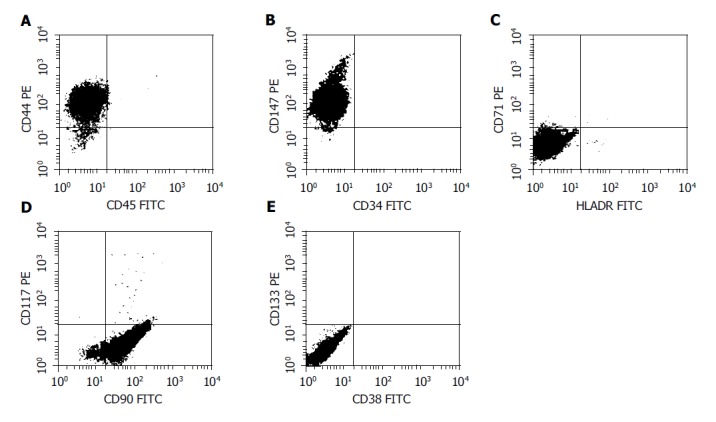
FACS analysis of the surface marker in the nestin-positive cells. The cells were labeled with FITC- or PE-conjugated antibodies and then analyzed in a flow cytometer. CD44, CD90 and CD147 were positive, whereas CD34, CD38, CD45, CD71, CD117, CD133 and HLA-DR were negative in the cells, resembling the phenotype of mesenchymal stem cells.
In vitro differentiation of the nestin-positive pancreatic progenitor cells
To investigate the ability of the nestin-positive cells to differentiate into pancreatic islet endocrine cells, the nestin-positive cells were cultured in the serum-free media with the cocktail of several growth factors for 6 d. RT-PCR analysis showed that the mRNA expression of insulin, glucagon and PDX-1 appeared, whereas the expression of nestin and Ngn3 disappeared in these cells after the induction culture (Figure 4). Furthermore, the intracellular insulin protein content was detectable in the cells after the induction culture, but not in the cells before the induction culture (Figure 5).
Figure 4.
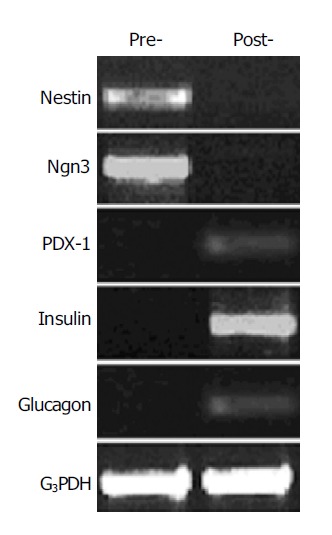
RT-PCR analysis of gene expression change in the nestin-positive cells before and after induction by using the primers for nestin (549 bp), Ngn3 (420 bp), PDX-1 (262 bp), insulin (261 bp), and glucagon (236 bp). G3PDH were used as internal control (452 bp). Pre-, cells before induction; post-, cells after induction.
Figure 5.
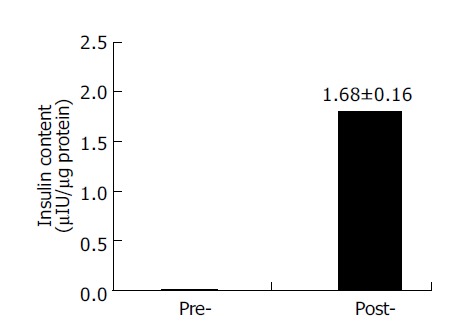
RIA detection of intra-cellular insulin content before and after induction. Data are the mean±SD of three separate experiments with the nestin-positive cells. Pre-, the cells before induction; post-, the cells after induction.
DISCUSSION
The intermediate filament protein nestin has been identified as a marker for neural stem cells[11,12]. A recent study showed that nestin-positive cells isolated from adult human and rat islets could be expanded and differentiated into pancreatic endocrine, exocrine and hepatic phenotypes in vitro, leading to the suggestion that they were multipotent tissue stem cells[2]. Studies have also shown that ES cell-derived cultures enriched for nestin-expressing cells can be differentiated in vitro into cells that produce both insulin and glucagon[10,13,14]. Furthermore, another paper demonstrated recently that nestin-positive precursors derived from human fetal pancreas were induced to differentiate into pancreatic endocrine cells that displayed the ability to reverse hyperglycemia in diabetic mice[3]. These observations have led to the hypothesis that nestin is a marker of pancreatic stem cells.
An important property of stem cells is their ability for self-renewal and differentiation into specific cell lineages. In the present study, we showed that nestin-positive cells isolated from human fetal pancreas had highly proliferative potential. These cells could be passaged more than 16 times in vitro. To determine whether the nestin-positive cells could differentiate into pancreatic endocrine cells, the cells were induced for differentiation after exposure to the serum-free media supplemented with the cocktail of growth factors. Our results revealed that the mRNA expression of pancreatic endocrine cell markers insulin, glucagon and PDX-1 was detected, whereas the expression of pancreatic progenitor cell markers nestin and Ngn3 disappeared in the cells after the induction. Moreover, the intracellular insulin protein content was also detectable in the cells after the induction. These results suggest that the nestin-positive pancreatic cells possess the characteristics of stem cells.
ABCG2 known as a member of ATP binding cassette (ABC) transporter superfamily, also called Bcrp1, can efflux the fluorescent dyes such as Hoechst 33342 from the cells termed as side population (SP) cells displaying low Hoechst fluorescence. SP cells were firstly isolated following Hoechst 33342 staining from murine hematopoietic stem cells, which was highly replicating and presented in the bone marrow of all species examined[15,16]. Soon after, it has been demonstrated that stem cells from skeletal muscle[17,18], brain[19], heart[20], lung[21] and possibly other tissues[22,23], as well as ES cells[23], can be identified by the SP phenotype. Therefore, the SP phenotype might represent a common molecular feature for stem cells possessing multi-organ plasticity[23]. Meanwhile, the studies have shown that ABCG2 gene expression alone defines the SP phenotype and is a conserved feature of stem cells from a wide variety of sources[23]. RT-PCR analysis in this study revealed that ABCG2 mRNA was expressed in the nestin-positive cells isolated from human fetal pancreas. These results further support that the nestin-positive cells have the characteristics of pancreatic stem cells.
Importantly, this study also showed that the nestin-positive pancreatic progenitor cells shared many phenotypic markers with mesenchymal stem cells derived from bone marrow. Mesenchymal stem cells of bone marrow origin are a kind of multipotential stem cells that can differentiate into adipocytic, chondrocytic or osteocytic lineages, neurons and functional hepatocyte-like cells under the appropriate induction condition[24-26]. Several other studies reported that mesenchymal stem cells were isolated from postnatal murine muscle and brain, human first-trimester fetal blood and liver, human umbilical cord, and human trabecular bone[27-30]. Therefore, it has been proposed that mesenchymal stem cells may be the universal stem cells akin to ES cells existing in multiple tissues, which take part in tissue repairs and regeneration and possess multi-organ plasticity. Interestingly, a recent paper shows that bone marrow harbors cells which have the capacity to differentiate into functionally competent pancreatic islet β-cells[31].
In summary, the nestin-positive cells isolated from human fetal pancreas possess the characteristics of pancreatic progenitor cells since they have highly proliferative potential and the capability of differentiation into pancreatic endocrine cells in vitro. We show for the first time that these nestin-positive pancreatic progenitor cells share many phenotypic markers with mesenchymal stem cells derived from bone marrow.
ACKNOWLEDGMENTS
This work was supported by the grants from the National Natural Science Foundation of China (30170443), Major State Basic Research Development Program of China (2001CB510105) and “211” Project Foundation of Peking University. We are grateful to Ai-Li Lu and Lu Zhang for excellent technical assistance.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30170443; Major State Basic Research Development Program of China, No. 2001CB510105 and “211” Project Foundation of Peking University
Co-correspondents: Ling-Song Li
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Tang X. Phenotypic determination and characterization of nestin-positive precursors derived from human fetal pancreas. Lab Invest. 2003;83:539–547. doi: 10.1097/01.lab.0000062890.40534.1c. [DOI] [PubMed] [Google Scholar]
- 4.Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 5.Treutelaar MK, Skidmore JM, Dias-Leme CL, Hara M, Zhang L, Simeone D, Martin DM, Burant CF. Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes. 2003;52:2503–2512. doi: 10.2337/diabetes.52.10.2503. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey RK, Bucay N, Beattie GM, Lopez A, Messam CA, Cirulli V, Hayek A. Characterization and isolation of promoter-defined nestin-positive cells from the human fetal pancreas. Diabetes. 2003;52:2519–2525. doi: 10.2337/diabetes.52.10.2519. [DOI] [PubMed] [Google Scholar]
- 7.Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92:1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beattie GM, Rubin JS, Mally MI, Otonkoski T, Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes. 1996;45:1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- 9.Hong TP, Andersen NA, Nielsen K, Karlsen AE, Fantuzzi G, Eizirik DL, Dinarello CA, Mandrup-Poulsen T. Interleukin-18 mRNA, but not interleukin-18 receptor mRNA, is constitutively expressed in islet beta-cells and up-regulated by interferon-gamma. Eur Cytokine Netw. 2000;11:193–205. [PubMed] [Google Scholar]
- 10.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 11.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 12.Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103(Pt 2):589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- 13.Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 17.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 19.Hulspas R, Quesenberry PJ. Characterization of neurosphere cell phenotypes by flow cytometry. Cytometry. 2000;40:245–250. [PubMed] [Google Scholar]
- 20.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 21.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 22.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–1345. doi: 10.1016/s0301-472x(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mosca JD, McIntosh KR. Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol. 2000;251:3–11. doi: 10.1007/978-3-642-57276-0_1. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 28.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 29.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 30.Sottile V, Halleux C, Bassilana F, Keller H, Seuwen K. Stem cell characteristics of human trabecular bone-derived cells. Bone. 2002;30:699–704. doi: 10.1016/s8756-3282(02)00674-9. [DOI] [PubMed] [Google Scholar]
- 31.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]


