Abstract
AIM: To evaluate the diagnostic value of different indirect methods like biochemical parameters, ultrasound (US) analysis, CT-scan and MRI/MRCP in comparison with endoscopic retrograde cholangiography (ERC), for diagnosis of biliary complications after liver transplantation.
METHODS: In 75 patients after liver transplantation, who received ERC due to suspected biliary complications, the result of the cholangiography was compared to the results of indirect imaging methods performed prior to ERC. The cholangiography showed no biliary stenosis (NoST) in 25 patients, AST in 27 and ITBL in 23 patients.
RESULTS: Biliary congestion as a result of AST was detected with a sensitivity of 68.4% in US analysis (specificity 91%), of 71% in MRI (specificity 25%) and of 40% in CT (specificity 57.1%). In ITBL, biliary congestion was detected with a sensitivity of 58.8% in the US, 88.9% in MRI and of 83.3% in CT. However, as anastomotic or ischemic stenoses were the underlying cause of biliary congestion, the sensitivity of detection was very low. In MRI detected the dominant stenosis at a correct localization in 22% and CT in 10%, while US failed completely. The biochemical parameters, showed no significant difference in bilirubin (median 5.7; 4,1; 2.5 mg/dL), alkaline phosp-hatase (median 360; 339; 527 U/L) or gamma glutamyl transferase (median 277; 220; 239 U/L) levels between NoST, AST and ITBL.
CONCLUSION: Our data confirm that indirect imaging methods to date cannot replace direct cholangiography for diagnosis of post transplant biliary stenoses. However MRI may have the potential to complement or precede imaging by cholangiography. Optimized MRCP-processing might further improve the diagnostic impact of this method.
Keywords: ERCP, Liver transplantation, Biliary strictures, Endoscopy, Therapy, Ultrasound, MRCP, Diagnosis
INTRODUCTION
Biliary tract complications after liver transplantation occur with a reported incidence of up to 34% and show a mortality of 5%[1-5]. The leading etiological factors are ischemic type biliary lesions (ITBL) and anastomotic strictures (AST) of duct-to-duct anastomosis, which may lead to malfunction and loss of the graft[6-10]. In addition leaks or stones in the biliary tract have to be considered[1,5,6]. There are several reports of successful endoscopic treatment of post transplant biliary stenoses (PTBS)[5-10]. However, the exact diagnosis and localization of PTBS prior to ERC is difficult. This may be due to several reasons: (1) Dilatation of the biliary system in transplanted livers may develop slower, (2) the biliary system may be filled with epithelial cast, which cannot be visualized by indirect imaging, (3) elevated liver enzymes and cholestasis parameters may be due to graft rejection or recurrence of the underlying pretransplant liver disease.
Therefore, the aim of this study was to evaluate the diagnostic value of different indirect methods such as bioch-emical parameters, ultrasound (US) analysis, CT-scan and MRI/MRCP in comparison with direct endoscopic retrograde cholangiography (ERC), which is still the gold standard for diagnosis of biliary complications after liver transplantation.
MATERIALS AND METHODS
Seventy-five patients (39 male, 36 female, median age 51 years [range 20-66 years]) received ERC due to suspected biliary complications after liver transplantation. In all patients liver biopsy was obtained prior to ERC for exclusion of graft rejection.
Biochemical cholestasis parameters like serum bilirubin level, alkaline phosphatase and gamma glutamyl transferase were determined at the time of indication for ERC. For analysis, cholestasis parameters, US reports, magnetic resonance imaging and computed tomography scan reports as well as ERC reports were put into a computer database (Access 2000, Microsoft Inc.).
According to the results of direct biliary imaging by ERC, patients were divided into three groups: Group A with no apparent stenosis of the biliary tract, classified as no stenosis (NoST); group B with a short stenosis in the anastomotic region, classified as AST; and group C with one or multiple non-AST of the biliary tract, classified as ITBL. ITBL was subdivided into three groups as proposed by Hintze[5] with regard to the localization of stenoses: Type I extrahepatic lesion, Type II intrahepatic lesion, Type III extra- and intrahepatic lesions.
Statistical analysis
Statistical analysis was performed with SPSS for Windows® release 11.0.1 (SPSS Inc.). For statistical analysis of qualitative characteristics, we used χ2 or Fisher’s exact test, when appropriate. To evaluate the effect of continuous variables we used the Mann-Whitney U test.
RESULTS
ERC showed no biliary stenosis (NoST) in 25 patients, AST in 27 and ITBL in 23 patients. The three groups showed no significant differences in age and gender with the exception of a predominance of women. There was a significant difference with regard to the underlying liver disease, as acute liver failure (ALF) was found significantly more often in ITBL patients (Table 1 and Figure 1).
Table 1.
Patient characteristics.
| NoST | AST | ITBL | |
| n | 25 | 27 | 23 |
| m/f | 16/9 | 11/16 (P = 0.081) | 13/10 (P = 0.401) |
| Median age (yr) | 50 yr [33-63] | 54 yr [33-65] (P = 0.09) | 49 yr [27-63] (P = 0.94) |
| Type of LTx | OLTx = 17/25 | OLTx = 17/27 | OLTx = 20/23 |
| LDLTx = 5/25 | (P = 0.461) | (P = 0.111) | |
| Split = 3/25 | LDLTx = 7/27 (P = 0.431) | LDLTx = 3/23 (P = 0.401) | |
| Split = 3/27 (P = 0.701) | Split = 0/23 (P = 0.131) |
NoST= no apparent biliary stenosis in ERC. AST= anastomotic stricture in ERC. ITBL= ischemic type biliary lesions in ERC. OLTx= orthotopic liver transplantation; LDLTx= Living donor liver transplantation.
Fisher’s exact test.
Figure 1.
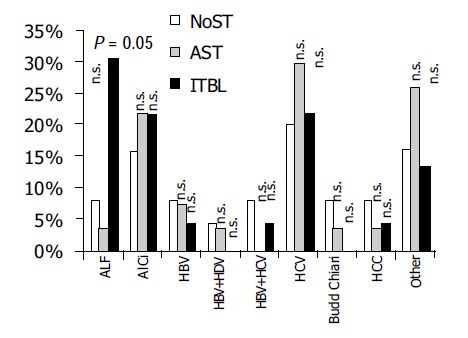
Differences in underlying liver disease prior to transplantation. NoST = no apparent biliary stenosis in ERC. AST = anastomotic stricture in ERC. ITBL = ischemic type biliary lesions in ERC. ALF = acute liver failure; AlCi = alcoholic liver cirrhosis; HBV = chronic hepatitis B virus infection; HDV = chronic hepatitis D virus superinfection; HCV = chronic hepatitis C virus infection; HCC = hepatocellular carcinoma; n.s. = not significant.
US were available in 88% of patients with NoST and in 74% of patients with AST or ITBL, respectively. MRI of the liver was performed in 16% of NoST, in 26% of AST and in 48% of ITBL patients. A CT-scan was available in 28% of NoST, in 19% of AST and in 26% of ITBL patients (Figure 2).
Figure 2.
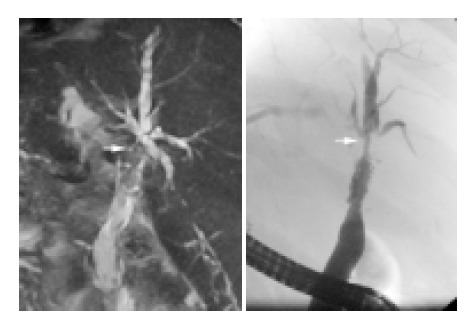
Comparison of ERC and MRC in posttransplant biliary stenosis. ITBL type III with hilar stenosis (arrow) and multiple peripheral duct stenoses. Left: MRC, right: ERC. The dominant hilar stenosis (arrow) is seen with both methods. The peripheral stenoses are better seen in ERC.
Biliary congestion as a result of AST was detected with a sensitivity of 68.4% in US analysis (specificity 91%), 71.4% in MRI (specificity 25%) and 40% in CT (specificity 57.1%). In ITBL, biliary congestion was detected with a sensitivity of 58.8% in US, 88.9% in MRI and of 83.3% in CT. However, as anastomotic or ischemic stenoses were the underlying cause of biliary congestion, the sensitivity and specificity as well as the correct localization of stenoses was very low. In AST MRI detected the dominant stenosis in 38.9% and CT in 10%, while US failed completely. In ITBL, MRI localized stenoses correctly in 22%, whereas US or CT failed (Table 2).
Table 2.
Impact of indirect imaging tools on diagnosis of posttransplant biliary strictures.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| US-biliary congestion all | 63.9 | 91.0 | 92.0 | 60.6 |
| -biliary congestion AST | 68.4 | 91.0 | 86.7 | 76.9 |
| -biliary congestion ITBL | 58.8 | 91.0 | 83.3 | 74.1 |
| US-biliary stenosis | 0 | 0 | 0 | 0 |
| US-concomitant biliary stones all | 22.0 | 97.0 | 66.7 | 82.5 |
| -stones AST | 0 | 97 | 0 | 91.7 |
| -stones ITBL | 0 | 97 | 0 | 94 |
| CT-biliary congestion all | 63.6 | 57.1 | 70.0 | 50.0 |
| -biliary congestion AST | 40.0 | 57.1 | 40.0 | 57.1 |
| -biliary congestion ITBL | 83.3 | 57.1 | 62.5 | 80.0 |
| CT-biliary stenosis | 10 | 0 | 0 | 43.75 |
| CT-concomitant biliary stones | 0 | 0 | 0 | 0 |
| MR-biliary congestion all | 83.3 | 25.0 | 83.3 | 25.0 |
| -biliary congestion AST | 71.4 | 25.0 | 62.5 | 33.3 |
| -biliaty congestion ITBL | 88.9 | 25.0 | 72.7 | 50.0 |
| MR-biliary stenosis | 38.9 | 75 | 87.5 | 21.4 |
| MR-correct localisation of stenosis | 22 | 75 | 80 | 17.6 |
| MR-concomitant biliary stones all | 33.0 | 94 | 50 | 89 |
| -stones AST | 0 | 94 | ||
| -stones ITBL | 0 | 94 |
PPV, positive predictive value; NPV, negative predictive value.
The presence of biliary stones were regularly detected in patients without stenosis with all indirect diagnostic methods but interestingly, they could almost never be visualized in patients with underlying anastomotic or ischemic type stenoses (Table 2).
Regarding the biochemical parameters, there was no significant difference in bilirubin (median 5.7; 4.1; 2.5 mg/dL), alkaline phosphatase (median 360; 339; 527 U/L) or gamma glutamyl transferase (median 277; 220; 239 U/L) levels between NoST, AST and ITBL (Table 3).
Table 3.
Impact of biochemical cholestasis parameters on diagnosis of post-transplant biliary strictures1.
| NoST | AST | ITBL | |
| Serum bilirubin | 5.7 (0.8-30) | 4.1 (0.7-9.5) | 2.5 (0.5-23.7) |
| (mg/dL) | P = 0.1792 | P = 0.2452 | |
| Alkaline | 360 (157-1206) | 339 (72-2185) | 527 (288-2256) |
| phosphatase (U/L) | P = 0.562 | P = 0.2392 | |
| Gamma glutamyl | 277 (64-832) | 220 (33-1580) | 239 (65-849) |
| tranferase (U/L) | P = 0.062 | P = 0.6692 |
1Data given as median and (range);
Mann-Whitney test.
DISCUSSION
Biliary strictures after liver transplantation are a diagnostic and therapeutic challenge. They occur in up to 34% of patients receiving liver transplantation[1-5]. Usually they appear 3-5 mo after transplantation and therefore easy direct imaging via post surgical t-tube is not possible.
Elevation of liver enzymes in patients after liver transplantation are caused by a variety of reasons, such as graft rejection, recurrence of the underlying liver disease, biliary strictures and/or biliary stones. As endoscopic retrograde or percutaneous transhepatic cholangiography for diagnostic reasons are invasive and technically often challenging, it would be desirable to have another easy diagnostic tool for differentiation between these causes of cholestasis. Therefore, the aim of this study was to evaluate the diagnostic value of these different indirect methods in comparison with (ERC), which is the gold standard for diagnosis of biliary complications after liver transplantation.
It is reported, that the level of alkaline phosphatase may be a diagnostic feature of ischemic biliary strictures[5]. We could not find any statistically significant difference of alkaline phosphatase, gamma glutamyl transferase or bilirubin serum level in patients with anastomotic or ischemic type biliary strictures compared to patients with a normal biliary system. Therefore biochemical liver function tests may provide a hint for the existence of PTBS but do not contribute to prove the case for or disclose the kind of PTBS.
In the ITBL group our study demonstrated a significantly higher proportion of patients with ALF than in both other groups as the underlying cause for transplantation. The reason therefore remains to be resolved. Several pathogenetic reasons such as thrombosis of the hepatic artery, cytomegaly virus infection, hepatitis C infection, etc. have been suggested as a cause of ITBL, however ALF as a risk factor for ITBL has not yet been demonstrated[12]. Further studies will have to clarify that issue.
In contrast to patients without transplantation, US is reported to have a low sensitivity (close to 50%) for the diag-nosis of posttransplant biliary congestion and strictures[13-15]. In contrast, Hussaini et al[16], reported a high negative predictive value of 95% for transabdominal US in the diagnosis of biliary tract complications, when statistically adjusted for the low prevalence rate of biliary complications in their post liver transplant patients. They assume that a normal ultrasonography makes the presence of biliary complications unlikely. In contrast, we observed a considerable proportion of patients with normal US despite of substantial bile duct stenosis. The specific localization of stenosis could not be visualized by ultrasonography. This may be due to the fact, that the post transplant bile ducts are often filled with epithelial cast, which could mask the true biliary diameter. Other authors presume that acute occlusion may not result in a prompt dilatation of the prestenotic bile ducts[13-15].
It is reported, that helical CT, which is often used to examine suspected vascular disease, can also demonstrate associated biliary complications[17], although exact data are not available. In our study, CT-scan was able to detect biliary congestion in 40% of AST and in 83% of ITBL with a specificity of 71%. The specific site of stenosis could be detected in 10% of patients.
Sensitivity and specificity of MR-imaging of post-transplant biliary complications is reported to be high[18-22]. For instance, Borasci et al[18], reported a sensitivity of 93%, specificity of 92%, a positive predictive value of 86% and a negative predictive value of 96% in detecting post-LTx biliary complications. Unfortunately most of the other MR-imaging studies did not compare MRI with direct cholangiography. In view of our data, the results of all these studies appear questionable[19-23].
Using MR-Imaging (MRI) in combination with MR-cholangiography (MRC) in our study sensitivity of correct diagnosis was highest regarding all indirect techniques. MRC techniques vary considerably from study to study and so, most studies are not comparable. There are novel computation modes under evaluation, which might improve the diagnostic impact of MRC in PTBS.
Therefore, to date MRC seems to be the most promising indirect tool for the diagnosis of biliary congestion in post liver transplant patients, but sensitivity of the specific type and pattern of stenoses still remains poor. However, with further improvement MRC may become a valuable tool before endoscopic intervention.
In conclusion, our data confirm that indirect imaging methods to date cannot replace direct cholangiography for diagnosis of PTBS. However MRI may have the potential to complement or precede imaging by cholangiography. Optimized MRC-processing might further improve the diagnostic impact of this method.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Li S, Stratta RJ, Langnas AN, Wood RP, Marujo W, Shaw BW. Diffuse biliary tract injury after orthotopic liver transplantation. Am J Surg. 1992;164:536–540. doi: 10.1016/s0002-9610(05)81196-1. [DOI] [PubMed] [Google Scholar]
- 2.Gholson CF, Zibari G, McDonald JC. Endoscopic diagnosis and management of biliary complications following orthotopic liver transplantation. Dig Dis Sci. 1996;41:1045–1053. doi: 10.1007/BF02088217. [DOI] [PubMed] [Google Scholar]
- 3.Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sossenheimer M, Slivka A, Carr-Locke D. Management of extrahepatic biliary disease after orthotopic liver transplantation: review of the literature and results of a multicenter survey. Endoscopy. 1996;28:565–571. doi: 10.1055/s-2007-1005556. [DOI] [PubMed] [Google Scholar]
- 5.Hintze RE, Abou-Rebyeh H, Adler A, Veltzke W, Langrehr J, Wiedenmann B, Neuhaus P. Endoscopic therapy of ischemia-type biliary lesions in patients following orthotopic liver transplantation. Z Gastroenterol. 1999;37:13–20. [PubMed] [Google Scholar]
- 6.Hintze RE, Adler A, Veltzke W, Abou-Rebyeh H, Felix R, Neuhaus P. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepatogastroenterology. 1997;44:258–262. [PubMed] [Google Scholar]
- 7.Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, Kwo P, Lehman GA. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224–231. doi: 10.1067/mge.2002.120813. [DOI] [PubMed] [Google Scholar]
- 8.Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003;23:156–162. doi: 10.1034/j.1600-0676.2003.00823.x. [DOI] [PubMed] [Google Scholar]
- 9.Morelli J, Mulcahy HE, Willner IR, Cunningham JT, Draganov P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374–379. doi: 10.1067/s0016-5107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 10.Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55–63. doi: 10.1067/mge.2000.106687. [DOI] [PubMed] [Google Scholar]
- 11.Mosca S, Militerno G, Guardascione MA, Amitrano L, Picciotto FP, Cuomo O. Late biliary tract complications after orthotopic liver transplantation: diagnostic and therapeutic role of endoscopic retrograde cholangiopancreatography. J Gastroenterol Hepatol. 2000;15:654–660. doi: 10.1046/j.1440-1746.2000.02198.x. [DOI] [PubMed] [Google Scholar]
- 12.Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885–890. doi: 10.1034/j.1600-6143.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AS, Ryan SM, Beese RC, Sidhu PS. Ultrasound of non-vascular complications in the post liver transplant patient. Clin Radiol. 2003;58:672–680. doi: 10.1016/s0009-9260(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 14.Zemel G, Zajko AB, Skolnick ML, Bron KM, Campbell WL. The role of sonography and transhepatic cholangiography in the diagnosis of biliary complications after liver transplantation. AJR Am J Roentgenol. 1988;151:943–946. doi: 10.2214/ajr.151.5.943. [DOI] [PubMed] [Google Scholar]
- 15.Kok T, Van der Sluis A, Klein JP, Van der Jagt EJ, Peeters PM, Slooff MJ, Bijleveld CM, Haagsma EB. Ultrasound and cholangiography for the diagnosis of biliary complications after orthotopic liver transplantation: a comparative study. J Clin Ultrasound. 1996;24:103–115. doi: 10.1002/(SICI)1097-0096(199603)24:3<103::AID-JCU1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Hussaini SH, Sheridan MB, Davies M. The predictive value of transabdominal ultrasonography in the diagnosis of biliary tract complications after orthotopic liver transplantation. Gut. 1999;45:900–903. doi: 10.1136/gut.45.6.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quiroga S, Sebastià MC, Margarit C, Castells L, Boyé R, Alvarez-Castells A. Complications of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics. 2001;21:1085–1102. doi: 10.1148/radiographics.21.5.g01se061085. [DOI] [PubMed] [Google Scholar]
- 18.Boraschi P, Braccini G, Gigoni R, Sartoni G, Neri E, Filipponi F, Mosca F, Bartolozzi C. Detection of biliary complications after orthotopic liver transplantation with MR cholangiography. Magn Reson Imaging. 2001;19:1097–1105. doi: 10.1016/s0730-725x(01)00443-x. [DOI] [PubMed] [Google Scholar]
- 19.Linhares MM, Gonzalez AM, Goldman SM, Coelho RD, Sato NY, Moura RM, Silva MH, Lanzoni VP, Salzedas A, Serra CB, et al. Magnetic resonance cholangiography in the diagnosis of biliary complications after orthotopic liver transplantation. Transplant Proc. 2004;36:947–948. doi: 10.1016/j.transproceed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Fulcher AS, Turner MA. Orthotopic liver transplantation: evaluation with MR cholangiography. Radiology. 1999;211:715–722. doi: 10.1148/radiology.211.3.r99jn17715. [DOI] [PubMed] [Google Scholar]
- 21.Meersschaut V, Mortelé KJ, Troisi R, Van Vlierberghe H, De Vos M, Defreyne L, de Hemptinne B, Kunnen M. Value of MR cholangiography in the evaluation of postoperative biliary complications following orthotopic liver transplantation. Eur Radiol. 2000;10:1576–1581. doi: 10.1007/s003300000379. [DOI] [PubMed] [Google Scholar]
- 22.Laghi A, Pavone P, Catalano C, Rossi M, Panebianco V, Alfani D, Passariello R. MR cholangiography of late biliary complications after liver transplantation. AJR Am J Roentgenol. 1999;172:1541–1546. doi: 10.2214/ajr.172.6.10350286. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Siegelman ES, Stolpen AH, Mitchell DG. MR imaging of complications after liver transplantation. AJR Am J Roentgenol. 2000;175:1145–1149. doi: 10.2214/ajr.175.4.1751145. [DOI] [PubMed] [Google Scholar]


