Abstract
AIM: To assess whether the molecular markers of malignant tumors could improve the understanding of tumor charact-eristics, and to observe the characteristics of expression of cell cycle markers Ki-67 and cyclin A in esophageal carcinoma and to analyze the relationship between proliferative activity of cancer cells and clinicopathological factors.
METHODS: Seventy of surgically resected esophageal squamous cell carcinoma (SCC) were examined by immun-ohistochemistry utilizing commercially available antibodies. Nuclear staining was regarded as a positive result. At least 50 fields in each tumor and non-tumor section were evaluated at a medium power (×200) to determine the proportion of tumor cells and the staining intensity of nuclei in the entire sections.
RESULTS: Ki-67 and cyclin A were only expressed in base cells of normal esophageal mucosa. The positive immuno-staining of nuclei of SCC was significantly higher than that in normal esophageal mucosa (t = 13.32 and t = 7.52, respectively, P<0.01). The distribution of positively stained was more diffuse and stronger in poorly differentiated SCC. Both Ki-67 and cyclin A expressions were related to histological grades of tumors (t = 3.5675 and t = 3.916; t = 2.13, respectively, P<0.05) but not to the sex and age of the patients, tumor size, lymphatic invasion, location, or stage grouping.
CONCLUSION: The proliferative activity of cancer cells may be understood by immunohistochemistry of Ki-67 and cyclin A in Chinese patients with esophageal SCC. These cell cycle markers may serve as an indicator of cancer cell proliferation rate. The overexpression of cell cycle markers Ki-67 and cyclin A suggests the poor SCC differentiation in patients with esophageal carcinoma.
Keywords: Proliferative activity, Esophageal neoplasms, Ki-67, Cyclin A
INTRODUCTION
Tumor cell cycle analysis has indicated that tumors with a higher proliferation rate show more aggressive clinical behaviors compared to tumors with a low proliferation rate[1]. Immuno-staining studies using antibodies that recognize the Ki-67 nuclear antigen, a marker of cell proliferation, have provided a reliable method to characterize malignant tumors[1-3]. Ki-67 is present in all periods of the cell cycle except for G0[2]. Cyclin A is a cell cycle regulatory protein, which achieves its maximal level during the S and G2 phases. It is regarded as a regulator of the transition to mitosis[4].
Esophageal carcinoma is the sixth most common cancer in the world, and one of the most lethal tumors. Especially, there is a high morbidity and mortality for this disease in China, accounting for 54% of all new esophageal cancers in the world[5]. Several studies have focused on cell cycle regulatory proteins in this cancer, trying to thoroughly elucidate its relation to clinicopathologic features and prognosis of esophageal carcinoma. Ikeda et al[6], have found a significant correlation of Ki-67 expression to patient survival; Furihata et al[7], reported that patients with cyclin A immunopositivity had a significantly poorer survival rate in esophageal squamous cell carcinoma (SCC). Several studies have demonstrated poor prognosis patients with down-regulation of p27 in this carcinoma[8,9]. Nagasawa et al[10], reported that immunohistochemical examination of cyclin D1 expression could provide important prognostic information in univariate and multivariate analyses and might be necessary for determining therapeutic strategies for esophageal cancer. Other studies have found that the expression of cell cycle markers is associated with the response to chemoradiotherapy in esophageal SCC[11,12], which is a very useful discovery for clinical oncology. However, study of cell cycle markers (Ki-67 and cyclin A) in esophageal SCC has not been reported in Chinese patients. In the present study, the expression of Ki-67 and cyclin A by immunostaining was found to be associated with the clinical characteristics of Chinese patients with esophageal cancer.
MATERIALS AND METHODS
Patients and specimens
Seventy patients (54 males and 16 females) with esophageal SCC underwent a surgical resection at the Department of Thoracic Surgery, People’s Hospital of Taizhou (Taizhou Medical School, Yangzhou University) between May 1999 and December 2002. All patients were operated with subtotal or total esophagectomy and radical lymph node dissection. Histopathologic specimens were fixed in 10% buffered formalin, processed routinely, and embedded in paraffin. All specimens were obtained from patients who did not receive chemo- or radiotherapy prior to surgical resection. All hematoxylin-eosin stained sections were reviewed and re-examined by pathologists. The grade of tumor differentiation was determined according to the histologic classification of the World Health Organization[13]. Tumors were staged accor-ding to the TNM classification of the esophagus[14].
Antibodies
The following antibodies were used in this study: mouse monoclonal antibody anti-human Ki-67 (Novocastr a Laboratories Ltd, Newcastle, UK), rabbit polyclonal antibody anti-human cyclin A (H-432) (Lab Vision Corporation, Fremount, CA). The final diluted concentrations (in TBS containing 1% BSA) were for anti-Ki-67 1:200 and for anti-cyclin A 1:250.
Immunohistochemical staining
The specimens with adjacent noncancerous esophageal mucosa were cut into 4-5-μm-thick sections and mounted onto Superfrost Plus slides (Germany), deparaffinized with xylene, rehydrated with graded concentrations of ethanol. Endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol. The slides were washed twice in TBS buffer (10 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5) for 5 min. Before application of the primary antibody, a microwave antigen retrieval technique was used (at 700 W for 15 min in 10 mmol/L sodium citrate solution, pH 6.0), after washing twice with TBS, an aliquot of 100 µL blocking solution (TBS containing 1% BSA, 3% fetal calf serum, and 3% normal horse serum for mouse monoclonal antibody or 3% normal goat serum for rabbit polyclonal antibody) was applied to each section and incubated for 1 h at RT. Then, an aliquot of 100 µL primary antibody was applied to each section and incubated at 4 °C overnight. After being washed thrice with TBS, the secondary antibody (biotinylated horse antimouse or goat antirabbit IgG, Vector Laboratories, Inc., Burlingame, CA) was applied for 30 min at RT. Immun-ostaining was performed using the avidin-biotin-peroxidase complex technique (Vector Laboratories, Inc.). Immuno-reactions were visualized with 0.0067% diaminobenzidine (Sigma) as the substrate with 0.03% hydrogen peroxide in 100 mmol/L Tris-HCl buffer for 3 min. The sections were lightly counterstained in Harris hematoxylin solution for microscopic examination. Simultaneously, each section was incubated with nonimmune mouse IgG or rabbit IgG instead of the primary antibody for an internal negative control.
The immunostained specimens were analyzed by two independent pathologists. Nuclear staining was regarded as a positive result. At least 50 fields in each tumor and non-tumor section were evaluated at a medium power (×200) to determine the proportion of tumor cells and the staining intensity of nuclei in the entire sections. The tumor cellularity was scored from 1 to 6 based on the proportion of tumor cells and the staining intensity was scored from 0 to 6 according to the intensity and staining pattern of the section. The staining index (SI) was calculated by multiplying the cellularity and staining scores as described previously[15]. Finally, the SI for a section was calculated as the mean of the SIs of all fields examined in that section. To confirm the reproducibility of the result, all sections were scored twice: the highest score for each index and the highest score between the two observers were thus reported.
Statistical analysis
The mean follow-up period was too short (18 mo) to allow the comparison with the disease-free and the overall survival times of our patients. We performed statistical analysis to verify the possibility of an association between the different variables of the considered tumors (histological type and grading, evidence of metastasis, and Ki-67, cyclin A expression levels). The correlations between the expression of Ki-67, cyclin A proteins and the various clinicopathological factors considered were determined using the Student’s t test at the 5% level.
RESULTS
Expression pattern of Ki-67 and cyclin A in normal human esophageal mucosa and in esophageal SCC
Without specific primary antibody to Ki-67 or cyclin A, no staining was observed in esophageal specimens (Figures 1A and 1E). Ki-67 and cyclin A were only expressed in base cells of normal esophageal mucosa (Figures 1B and 1F). The staining of Ki-67 was confined to the nuclei of cells, while the staining of cyclin A was concentrated mainly on the nuclei of cells, occasionally, the cytoplasm was also stained. Ki-67 immunostaining was also confined to the nuclei of neoplastic cells. Ki-67 staining was observed in well- and moderately differentiated esophageal SCC (Figure 1C), but it was more diffuse and stronger in poorly differentiated SCC (Figure 1D). The distribution of positively stained cyclin A was similar to that of Ki-67 staining (Figures 1G and 1H).
Figure 1.
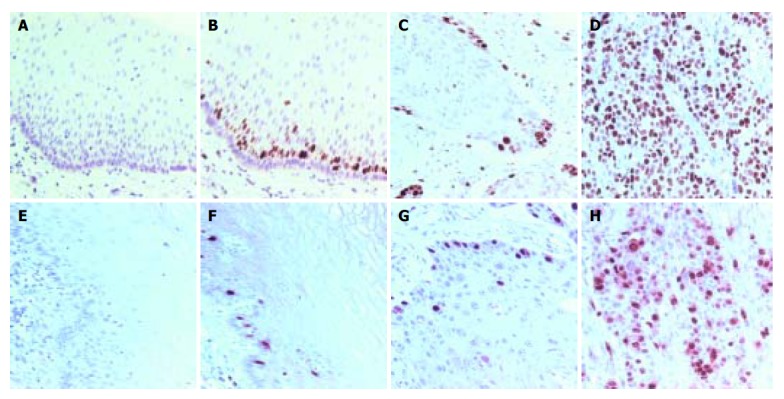
Ki-67 and cyclin A staining patterns in human normal esophageal mucosa and esophageal SCC. A and E: Negative control in normal esophageal mucosa; B and F: positive nuclear staining in normal esophageal mucosa; C and G: moderately differentiated SCC; D and H: poorly differentiated SCC. Positive nuclear staining was located in base cells in normal mucosa. The diffuse and strong Ki-67 immunostaining in esophageal SCC and the higher SI of the poorly differentiated SCC were found compared with the other carcinomas. Counterstaining with hematoxylin, ×200.
Ki-67 and cyclin A staining and clinicopathological factors
The correlations between the SIs of Ki-67, cyclin A and the clinicopathologic features of esophageal SCC are summarized in Table 1. The SIs of Ki-67 and cyclin A did not significantly correlate with the sex and age of patients or with tumor stage, but were significantly higher in carcinomas than in normal tissues (t = 13.32 and t = 7.52, respectively, P<0.01 for each protein).
Table 1.
Relationship between Ki-67, cyclinA and clinicopathologic characteristics of esophageal SCC (mean±SD).
| Factor | n |
SI |
|||
| Ki-67 | P | Cyclin A | P | ||
| Sex | |||||
| M | 54 | 23.5±5.9 | 13.9±6.3 | ||
| F | 16 | 22.3±6.8 | >0.05 | 12.0±6.1 | >0.05 |
| Age (yr) | |||||
| <60 | 29 | 23.1±6.2 | 13.8±6.9 | ||
| ≥60 | 41 | 22.1±6.9 | >0.05 | 12.5±6.3 | >0.05 |
| Histological | grade | ||||
| Normal | 20 | 3.9±2.1 | 2.8±1.2 | ||
| SCC | 70 | 22.6±6.1 | <0.01 | 13.1±6.2 | <0.01 |
| Well | 26 | 21.0±6.5 | 11.5±6.5 | ||
| Moderate | 24 | 20.3±5.9 | <0.05 | 13.0±6.8 | |
| Poor | 20 | 27.4±5.1 | <0.05 | 15.6±5.4 | <0.05 |
| Lymphatic | invasion | ||||
| (-) | 39 | 22.0±6.9 | 12.6±6.7 | ||
| (+) | 31 | 24.2±5.2 | >0.05 | 13.1±5.8 | >0.05 |
| Location | |||||
| Cervical | 1 | 24.1 | 13.3 | ||
| Upper | 8 | 23.4±6.2 | 12.7±4.9 | ||
| Middle | 46 | 22.0±7.1 | 10.9±6.4 | ||
| Lower | 15 | 22.3±4.9 | >0.05 | 12.1±5.0 | >0.05 |
| Stage | |||||
| 0 | 1 | 18.2 | 7.3 | ||
| I | 15 | 22.9±6.5 | 14.0±6.8 | ||
| II | 43 | 20.6±6.7 | 12.5±5.8 | ||
| III | 7 | 23.5±5.9 | 12.8±5.5 | ||
| IV | 4 | 24.8±6.2 | >0.05 | 13.1±6.0 | >0.05 |
Cervical: cervical esophagus; upper: upper intra-thoracic esophagus; middle: middle intra-thoracic esophagus; lower: lower intra-thoracic esophagus.
The Ki-67 SI ranged 7.7-34.5 (mean: 22.6±6.1). The index did not correlate significantly to the sex and age of patients, tumor size, lymph node metastasis, depth of invasion or tumor stage. However, the index was significantly higher in poorly differentiated SCC than in well- and moderately differentiated SCC (t = 3.5675 and t = 3.916, respectively, P<0.05).
The cyclin A SI ranged 3.2-29.2 (mean: 13.1±6.2) and was not significantly related to the sex and age of patients, tumor size, lymph node metastasis, depth of invasion or tumor stage. Compared to Ki-67, the cyclin A SI correlated with tumor differentiation and was significantly higher in poorly differentiated SCC than in well-differentiated SCC (t = 2.13, P<0.05).
DISCUSSION
The proliferative activity of esophageal SCC has been investigated by calculating the immunohistochemical index of cell proliferation using two cell cycle regulators, Ki-67 and cyclin A, as markers[6-10]. Because Ki-67 is present in proliferating cells but not in cells in the G0 phase, it may serve as an indicator of cancer cell proliferation rate. Our results suggested that the SI of Ki-67 correlated with differentiation of esophageal SCC but not with lymph node metastasis and stage of tumors. During the cell cycle progression, cyclin A is involved in the onset of DNA replication and is required for the G2-M transition. Overexpression of cyclin A would, therefore, contribute to the high proliferative activity in cancer cells. In our study, the SI of cyclin A was higher in esophageal SCC than in normal tissues (P<0.01), thus esophageal SCC exhibited overexpression of cyclin A. Several researchers have also demonstrated that the expression of cell cycle markers varies in esophageal precancerous lesions and cancer tissues, and these markers help distinguish high-grade dysplasia from mucosal invasive carcinoma[16]. The SI of cyclin A was also significantly higher in poorly differentiated SCC than in well-differentiated SCC, suggesting that cyclin A may reflect the high proliferative activity of cancer cells. The observation is concordant to results reported by Furihata et al[7], and Nozoe et al[17].
In conclusion, cell cycle regulatory proteins Ki-67 and cyclin A are expressed in esophageal SCC and associated with some clinicopathological features of Chinese patients. These biologic markers may improve the characterization of esophageal SCC.
Footnotes
Supported by the Key Medical Talent Foundation of Jiangsu Province, China, No. 2001-34 and 2002-15
References
- 1.Tubiana M, Courdi A. Cell proliferation kinetics in human solid tumors: relation to probability of metastatic dissemination and long-term survival. Radiother Oncol. 1989;15:1–18. doi: 10.1016/0167-8140(89)90113-8. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 3.Cattoretti G, Becker MH, Key G, Duchrow M, Schlüter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- 4.Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995;147:545–560. [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM, Läärä E, Muir CS. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988;41:184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda G, Isaji S, Chandra B, Watanabe M, Kawarada Y. Prognostic significance of biologic factors in squamous cell carcinoma of the esophagus. Cancer. 1999;86:1396–1405. doi: 10.1002/(sici)1097-0142(19991015)86:8<1396::aid-cncr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Furihata M, Ishikawa T, Inoue A, Yoshikawa C, Sonobe H, Ohtsuki Y, Araki K, Ogoshi S. Determination of the prognostic significance of unscheduled cyclin A overexpression in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 1996;2:1781–1785. [PubMed] [Google Scholar]
- 8.Shamma A, Doki Y, Tsujinaka T, Shiozaki H, Inoue M, Yano M, Kawanishi K, Monden M. Loss of p27(KIP1) expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Oncology. 2000;58:152–158. doi: 10.1159/000012092. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi Y, Sasano H, Yamaki H, Shizawa S, Shineha R, Akaishi T, Satomi S, Nagura H. Cell cycle inhibitory protein p27 in esophageal squamous cell carcinoma. Anticancer Res. 1999;19:1843–1848. [PubMed] [Google Scholar]
- 10.Nagasawa S, Onda M, Sasajima K, Makino H, Yamashita K, Takubo K, Miyashita M. Cyclin D1 overexpression as a prognostic factor in patients with esophageal carcinoma. J Surg Oncol. 2001;78:208–214. doi: 10.1002/jso.1152. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima Y, Miyake S, Tanaka K, Ogiya K, Toukairin Y, Kawada K, Nishikage T, Nagai K, Kawano T. The expressions of p21 and pRB may be good indicators for the sensitivity of esophageal squamous cell cancers to CPT-11: Cell proliferation activity correlates with the effect of CPT-11. Cancer Sci. 2004;95:464–468. doi: 10.1111/j.1349-7006.2004.tb03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi H, Ozawa S, Ando N, Kitagawa Y, Ueda M, Kitajima M. Cell-cycle regulators and the Ki-67 labeling index can predict the response to chemoradiotherapy and the survival of patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2003;10:792–800. doi: 10.1245/aso.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Sarbia M, Bittinger F, Porschen R, Dutkowski P, Willers R, Gabbert HE. Prognostic value of histopathologic parameters of esophageal squamous cell carcinoma. Cancer. 1995;76:922–927. doi: 10.1002/1097-0142(19950915)76:6<922::aid-cncr2820760603>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Iizuka T, Onoda T. TNM classification of carcinoma of the esophagus. Gan To Kagaku Ryoho. 1997;24:893–901. [PubMed] [Google Scholar]
- 15.King RJ, Coffer AI, Gilbert J, Lewis K, Nash R, Millis R, Raju S, Taylor RW. Histochemical studies with a monoclonal antibody raised against a partially purified soluble estradiol receptor preparation from human myometrium. Cancer Res. 1985;45:5728–5733. [PubMed] [Google Scholar]
- 16.Ohbu M, Kobayashi N, Okayasu I. Expression of cell cycle regulatory proteins in the multistep process of oesophageal carcinogenesis: stepwise over-expression of cyclin E and p53, reduction of p21(WAF1/CIP1) and dysregulation of cyclin D1 and p27(KIP1) Histopathology. 2001;39:589–596. doi: 10.1046/j.1365-2559.2001.01279.x. [DOI] [PubMed] [Google Scholar]
- 17.Nozoe T, Korenaga D, Futatsugi M, Saeki H, Ohga T, Sugimachi K. Cyclin A expression in superficial squamous cell carcinoma of the esophagus and coexisting infiltrated lymphocyte follicle. Cancer Lett. 2002;188:221–229. doi: 10.1016/s0304-3835(02)00434-2. [DOI] [PubMed] [Google Scholar]


