Abstract
AIM: To evaluate the relationship of expression of paxillin, syndecan-1 and EMMPRIN proteins with clinicopathological features in hepatocellular carcinoma (HCC).
METHODS: Fifty-one patients who underwent HCC resection were recruited in the study. Paxillin, syndecan-1 and EMMPRIN proteins in HCC tissues were detected with immunohistochemical staining.
RESULTS: Of 51 cases of HCC, 23 (45%) exhibited paxillin protein positive expression. Of 42 cases of adjacent non-tumor liver tissues, 24 (57%) exhibited positive expression. Positive paxillin protein expression was associated with low differentiation (r = 0.406, P = 0.004), with the presence of portal vein thrombosis (r = 0.325, P = 0.021), with extra-hepatic metastasis (r = 0.346, P = 0.014). Of 51 cases of HCC, 28 (55%) exhibited syndecan-1 protein positive expression. Of 42 cases of adjacent non-tumor liver tissues, 23 (55%) exhibited positive expression. Positive snydecan-1 protein expression was associated with well differentiation (r = 0.491, P = 0.001), with no extra-hepatic metastasis (r = 0.346, P = 0.014). Of 51 cases of HCC, 28 (55%) exhibited EMMPRIN protein positive expression. Of 42 cases of adjacent non-tumor liver tissues, 21 (50%) exhibited positive expression. Expression of EMMPRIN protein was not associated with serum AFP level, HBsAg status, presence of microsatellite nodule, tumor size, presence of cirrhosis and necrosis, differentiation, presence of portal vein thrombosis, extra-hepatic metastasis, disease-free survival and overall survival (P>0.05). Expression of paxillin protein was correlated conversely with the expression of syndecan-1 protein in HCC (r = -0.366, P = 0.010).
CONCLUSION: Expression of paxillin and syndecan-1 proteins in HCC may affect its invasive and metastatic ability of the tumor. There may be a converse correlation between the expression of paxillin and syndecan-1 protein in HCC. Expression of EMMPRIN protein may be detected in HCC, but it may play little role in the invasion and metastasis of HCC.
Keywords: Hepatocellular carcinoma, Paxillin, Syndecan-1, EMMPRIN, Immunohistochemistry
INTRODUCTION
Primary cancers of the liver in adults are of two main histological types: hepatocellular carcinoma (HCC) and cholangiocarcinoma. HCC is a frequently occurring tumor in individuals in many developing countries[1]. It ranks fifth in frequency worldwide among all malignancies and causes one million deaths annually[2], yet its incidence is increasing steadily in various countries[3-5]. Epidemiology studies showed that primary liver cancer is the second major cause of mortality in China[6] and it accounts for 53% of all liver cancer deaths worldwide[7]. Though with great development in diagnosis and therapy, the prognosis of patients with HCC remains dismal for its high rate of metastasis and recurrence. For patients in the advanced stages, the median survival is less than 6 mo, no matter what kinds of therapy were managed[8-12]. So it is urgent to further explore the mechanism of HCC occurrence, progress and metastasis.
Progression and metastasis are malignant characteristics of malignant tumors. It includes the cellular adhesion (between tumor cell and normal cell, also between tumor cells) and the destruction of extracellular matrix (ECM) in the progressive and metastatic process of malignant tumor. During the process, the molecules existing in ECM and the receptors or ligands existing on the surfaces of tumor cells play critical roles[13,14].
Focal adhesions form a structural link between the ECM and the actin cytoskeleton and are also important sites of signal transduction; their components propagate signals arising from the activation of integrins following their engagement with ECM proteins, such as fibronectin, collagen and laminin. Paxillin is a central protein within the focal adhesion[15]. Its primary function is as a molecular adapter or scaffold protein that provides multiple docking sites at the plasma membrane for an array of signaling and structural proteins. For example, it provides a platform for protein tyrosine kinases such as focal adhesion kinase (FAK) and SRC, which are activated as a result of adhesion or growth factor stimulation. Paxillin binds to many proteins that are involved in effecting changes in the organization of the actin cytoskeleton, which are necessary for cell motility events associated with embryonic development, wound repair and tumor metastasis[16,17].
Syndecans comprise a gene family of transmembrane proteoglycans that regulate cellular behavior through interactions with various effectors including heparin-binding growth factors and insoluble matrix components. Syndecan-1, a transmembrane heparin sulfate proteoglycan, localizes in epithelial cells and has been shown to be present in normal hepatocytes[18]. It interacts with growth factors, matrix components, and other extracellular proteins and is thought to be involved in processes such as cell growth, differentiation and adhesion. The expression of syndecan-1 appears generally downregulated in human carcinomas and in experimental cancer models, whereas transfectional expression of syndecan-1 in cultured cancer cells has been shown to inhibit their growth and other aspects of malignant behavior[19].
Extracellular matrix metalloproteinase inducer (EMMPRIN), which is also called CD147 and basigin, is a transmembrane glycoprotein with two immunoglobulin-like domains and forms a family with embigin and neuroplastin. EMMPRIN in tumor cells triggers the production or release of matrix metalloproteinases in the surrounding mesenchymal cells and tumor cells, thereby contributing to tumor invasion[20-23].
MATERIALS AND METHODS
Patients
Fifty-one patients (32 men and 19 women; mean age 51±11 years, range 24-69 years) who underwent resection of HCC in the Department of Surgery of Second Affiliated Hospital to Sun Yat-Sen University were studied. None of 51 patients had received any preoperative treatment. Classified according to the Chinese Diagnosis and Treatment Standard for Common Malignant Tumors, 35 carcinomas were well-differentiated (Edmonson grade 1-2) and 16 carcinomas were low-differentiated (Edmonson grade 3-4). Tissue specimens were fixed promptly with 100 g/L formaldehyde solution, embedded in paraffin and cut into 4 μm sections. Sections of 42 carcinomas were excised from the marginal part of the tumor containing both HCC and normal liver tissues.
Immunohistochemistry
Fifty-one patients, who underwent HCC resection, were recruited in the study. A 3-step immunoperoxidase technique, using the streptavidin-peroxidase (S-P), was employed for paxillin, syndecan-1 and EMMPRIN detection. All the sections were routinely deparaffinized and re-hydrated; then the sections were rinsed in phosphate-buffered saline (PBS, pH 7.4) and subsequently were treated for antigen retrieve. Sections for paxillin and syndecan-1 staining were treated in EDTA (1 mmol/L, pH 8.0) in water bath. Sections for EMMPRIN staining were treated in sodium citrate buffered saline (0.001 mol/L, pH 6.0) with microwave. After cooling at room temperature for 20 min, the sections were rinsed in PBS, then immersed in 3% H2O2 for 15 min to block the endogenous enzymes. After being rinsed in PBS, the sections were incubated with normal goat serum at 37 °C for 15 min to block nonspecific antibodies. After interaction with anti-paxillin antibody (No. 5H11, Maxim Biological and Technical Company, Fujian, China; ready-to-use), anti-syndecan-1 and anti-EMMPRIN antibodies (No.5F7 and polyclonal antibody, Zhongshan Biological and Technical Company, Beijing, China; diluted 1:70), the sections were rinsed in PBS, then incubated with biotinylated secondary antibodies and rinsed in PBS again. After interaction with streptavidin-HRP and being rinsed in PBS, the sections were visualized by reaction with 3,3’-diaminobenzidine and counter-stained with hematoxylin.
The determination, whether the tumor and the normal tissues were positive or not, was performed by two persons. A tumor or normal tissue, more than 10% of cancer cells or normal hepatic cells, stained with those antibodies was recognized as positive. The sections of lung carcinoma and bladder carcinoma tissues known for those antibodies stained positive were used as positive controls and normal goat serum and PBS substituting the primary antibody were used as negative controls.
Clinicopathological and follow-up data
Clinicopathological classification of the investigated HCC was made according to the criteria described by the Chinese Diagnosis and Treatment Standard for Common Malignant Tumors. Some clinicopathological findings (presence of cirrhosis and necrosis, histological grading and metastasis) were judged by two pathologists, others (presence of microsatellite nodule and tumor size) were judged by ultrasonic examination.
Postoperative follow-up included monitoring for disease recurrence by serum AFP level and chest X-ray detection, together with ultrasonography or computed tomography (CT) scan every 3 mo. Recurrence of tumor was diagnosed by the detection of any intra-hepatic or extra-hepatic tumor with a typical enhancement pattern of HCC in contrast CT scan and elevation of serum AFP level compared with the previous level. Disease-free survival was calculated from the date of hepatic resection to the date when recurrence was diagnosed or, in the absence of detectable recurrence, to the date of death or last follow-up.
Statistical analysis
Statistical analysis was performed by the generalized Wilcoxon’s test (or the Fisher’s exact test, where appropriate). Postoperative prognosis was evaluated with the Kaplan-Meier method and compared between groups by the log-rank test; the significance of the prognostic value of the variables was estimated with Cox’s multivariate proportional hazard model. All statistical analyses were performed using SPSS Win program package 8.0. Differences were considered as significant when the P value was less than 0.05.
RESULTS
Correlation between expression of paxillin protein and clinicopathological features in HCC
Of 51 cases that we assayed for paxillin protein expression in HCC, 23 (45%) exhibited positive expression (Figure 1A). Of 42 cases of adjacent non-tumor liver tissues, 24 (57%) exhibited positive expression. If paxillin expression was compared between HCC and the adjacent non-tumor liver tissues, there was no significant difference (P = 0.415). Positive paxillin expression was associated with low differentiation (r = 0.406, P = 0.004), with the presence of portal vein thrombosis (r = 0.325, P = 0.021), with extra-hepatic metastasis (r = 0.346, P = 0.014). Expression of paxillin protein was not associated with serum AFP level (P = 0.604), HBsAg status (P = 0.638), presence of microsatellite nodule (P = 0.991), tumor size (P = 0.272), presence of cirrhosis and necrosis (P = 0.886 and 0.922, respectively, Table 1).
Figure 1.
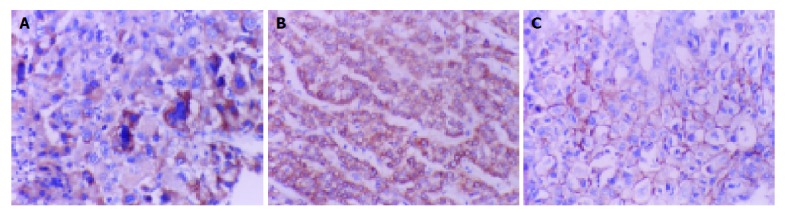
Immunohistochemical detection. A: Paxillin protein positive locates in cytoplasm of low-differentiated HCC cells (×200); B: Syndecan-1 protein positive locates in cytoplasm of well-differentiated HCC cells (×200); C: EMMPRIN protein positive locates in cytomembrane of well-differentiated HCC cells (×200).
Table 1.
Expression of paxillin protein categorized by pathological variables.
| Variables |
Paxillin |
P | |
| - | + | ||
| Serum AFP level | |||
| ≤25 ng/mL (n = 22) | 13 | 9 | 0.604 |
| >25 ng/mL (n = 29) | 15 | 14 | |
| HBsAg | |||
| Positive (n = 16) | 8 | 8 | 0.638 |
| Negative (n = 35) | 20 | 15 | |
| Microsatellite nodule | |||
| Absent (n = 31) | 17 | 14 | 0.991 |
| Present (n = 20) | 11 | 9 | |
| Tumor size | |||
| ≤3 cm (n = 18) | 8 | 10 | 0.272 |
| >3 cm (n = 33) | 20 | 13 | |
| Cirrhosis | |||
| Absent (n = 36) | 20 | 16 | 0.886 |
| Present (n = 15) | 8 | 7 | |
| Necrosis | |||
| Absent (n = 24) | 13 | 11 | 0.922 |
| Present (n = 27) | 15 | 12 | |
| Portal vein thrombosis | |||
| Absent (n = 37) | 24 | 13 | 0.021 |
| Present (n = 14) | 4 | 10 | |
| Differentiation | |||
| Well (n = 35) | 24 | 11 | 0.004 |
| Low (n = 16) | 4 | 12 | |
| Extra-hepatic metastasis | |||
| Absent (n = 41) | 26 | 15 | 0.014 |
| Present (n = 10) | 2 | 8 | |
Correlation between expression of syndecan-1 protein and clinicopathological features in HCC
Of 51 cases that we assayed for syndecan-1 protein expression in HCC, 28 (55%) exhibited positive expression (Figure 1B). Of 42 cases of adjacent non-tumor liver tissues, 23 (55%) exhibited positive expression. If syndecan-1 expression was compared between HCC and the adjacent non-tumor liver tissues, there was no significant difference (P = 0.842). Positive syndecan-1 expression was associated with well differentiation (r = 0.491, P = 0.001), with no extra-hepatic metastasis (r = 0.346, P = 0.014). Expression of syndecan-1 protein was not associated with serum AFP level (P = 0.280), HBsAg status (P = 0.465), presence of microsatellite nodule (P = 0.578), tumor size (P = 0.607), presence of cirrhosis and necrosis (P = 0.640 and 0.646, respectively), presence of portal vein thrombosis (P = 0.292). (Table 2).
Table 2.
Expression of syndecan-1 protein categorized by pathological variables.
| Variables |
Syndecan-1 |
P | |
| - | + | ||
| Serum AFP level | |||
| ≤25 ng/mL (n = 22) | 8 | 14 | 0.28 |
| >25 ng/mL (n = 29) | 15 | 14 | |
| HBsAg | |||
| Positive (n = 16) | 6 | 10 | 0.465 |
| Negative (n = 35) | 17 | 18 | |
| Microsatellite nodule | |||
| Absent (n = 31) | 13 | 18 | 0.578 |
| Present (n = 20) | 10 | 10 | |
| Tumor size | |||
| ≤3 cm (n = 18) | 9 | 9 | 0.607 |
| >3 cm (n = 33) | 14 | 19 | |
| Cirrhosis | |||
| Absent (n = 36) | 17 | 19 | 0.64 |
| Present (n = 15) | 6 | 9 | |
| Necrosis | |||
| Absent (n = 24) | 10 | 14 | 0.646 |
| Present (n = 27) | 13 | 14 | |
| Portal vein thrombosis | |||
| Absent (n = 37) | 15 | 22 | 0.292 |
| Present (n = 14) | 8 | 6 | |
| Differentiation | |||
| Well (n = 35) | 10 | 25 | 0.001 |
| Low (n = 16) | 13 | 3 | |
| Extra-hepatic metastasis | |||
| Absent (n = 41) | 15 | 26 | 0.014 |
| Present (n = 10) | 8 | 2 | |
Correlation between expression of EMMPRIN protein and clinicopathological features in HCC
Of 51 cases that we assayed for EMMPRIN protein expression in HCC, 28 (55%) exhibited positive expression (Figure 1C). Of 42 cases of adjacent non-tumor liver tissues, 21 (50%) exhibited positive expression. If EMMPRIN expression was compared between HCC and the adjacent non-tumor liver tissues, there was no significant difference (P = 0.842). Expression of EMMPRIN protein was not associated with serum AFP level (P = 0.242), HBsAg status (P = 0.284), presence of microsatellite nodule (P = 0.576), tumor size (P = 0.607), presence of cirrhosis and necrosis (P = 0.281 and 0.992, respectively), differentiation (P = 0.897), presence of portal vein thrombosis and extra-hepatic metastasis (P = 0.292 and 0.721, respectively). (Table 3).
Table 3.
Expression of EMMPRIN protein categorized by pathological variables.
| Variables |
EMMPRIN |
P | |
| - | + | ||
| Serum AFP level | |||
| ≤25 ng/mL (n = 22) | 12 | 10 | 0.242 |
| >25 ng/mL (n = 29) | 11 | 18 | |
| HBsAg | |||
| Positive (n = 16) | 9 | 7 | 0.284 |
| Negative (n = 35) | 14 | 21 | |
| Microsatellite nodule | |||
| Absent (n = 31) | 13 | 18 | 0.576 |
| Present (n = 20) | 10 | 10 | |
| Tumor size | |||
| ≤3 cm (n = 18) | 9 | 9 | 0.607 |
| >3 cm (n = 33) | 14 | 19 | |
| Cirrhosis | |||
| Absent (n = 36) | 18 | 18 | 0.281 |
| Present (n = 15) | 5 | 10 | |
| Necrosis | |||
| Absent (n = 24) | 11 | 13 | 0.992 |
| Present (n = 27) | 12 | 15 | |
| Portal vein thrombosis | |||
| Absent (n = 37) | 15 | 22 | 0.292 |
| Present (n = 14) | 8 | 6 | |
| Differentiation | |||
| Well (n = 35) | 16 | 19 | 0.897 |
| Low (n = 16) | 7 | 9 | |
| Extra-hepatic metastasis | |||
| Absent (n = 41) | 19 | 22 | 0.721 |
| Present (n = 10) | 4 | 6 | |
Prognostic value of paxillin, syndecan-1 and EMMPRIN proteins on disease-free survival and overall survival
Of 51 cases of HCC, median follow-up of the patients was 26 mo (range 5-90 mo). At the time of analysis, 21 of the 51 HCC patients had postoperative recurrence. The disease-free survival and overall survival were compared between two groups of patients who were segregated in positive and negative expression of those proteins. Patients with negative expression of EMMPRIN protein had better disease-free survival than those with positive expression, but the difference was not statistically significant (P = 0.087, Figure 2). The disease-free survival of the 51 HCC patients was not related to the expression of paxillin and syndecan-1 proteins (P = 0.350 and 0.654, respectively). The overall survival of those HCC patients was not related to the expression of paxillin, syndecan-1 and EMMPRIN proteins (P = 0.916, 0.538, and 0.476, respectively).
Figure 2.
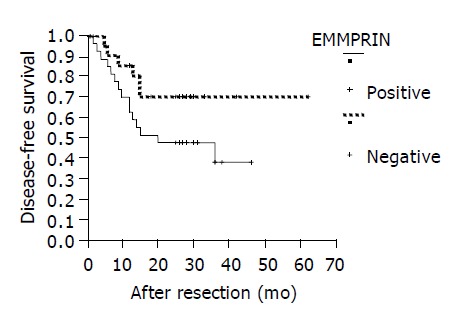
Disease-free analyses of HCC patients segregated into negative and positive expression of EMMPRIN protein (P = 0.087).
The expression of paxillin, syndecan-1 and EMMPRIN proteins was enabled to enter into a Cox’s regression analysis of disease-free survival and overall survival together with serum AFP level, HBsAg status, presence of microsatellite nodule, tumor size, presence of cirrhosis and necrosis, differentiation, presence of portal vein thrombosis and extra-hepatic metastasis. No significant prognostic factor was found for disease-free survival and overall survival (P>0.05, data not shown).
Correlation among expression of paxillin, syndecan-1 and EMMPRIN protein in HCC
Expression of paxillin protein was correlated conversely with the expression of syndecan-1 protein in HCC (r = -0.366, P = 0.010, Table 4). Expression of EMMPRIN protein was not correlated with the expression of paxillin and syndecan-1 protein in HCC (P = 0.184 and 0.141, respectively, Table 5).
Table 4.
Association between paxillin and syndecan-1 protein.
| Paxillin |
Syndecan-1 |
P | |
| - | + | ||
| Negative | 8 | 20 | 0.01 |
| Positive | 15 | 8 | |
Table 5.
Association between EMMPRIN and paxillin, syndecan-1 protein.
| Paxillin |
EMMPRIN |
P | |
| - | + | ||
| Negative | 15 | 13 | 0.184 |
| Positive | 8 | 15 | |
| Syndecan-1 | |||
| Negative | 13 | 10 | 0.141 |
| Positive | 10 | 18 | |
DISCUSSION
Evaluation of adhesion and proteolytic destruction of ECM and basement membranes (BM) has shown important clinical implications in the invasive and metastatic process of many types of malignant tumors. Immunohistochemical detection of factors attributed to adhesion and proteolytic destruction of ECM and BM might provide important prognostic values, independent of conventional pathological factors in cancer patients[24,25]. HCC is a highly invasive and metastatic malignancy. HCC quickly permeates the liver through the portal venous system; portal vein thrombosis is found at a high proportion in the advanced cases. Metastases to regional lymph nodes are also common. Paxillin, syndecan-1 and EMMPRIN proteins appear to be promising immunohistochemical markers that might have a value to evaluate malignancy in other types of cancers[17,21,26-29]. To our knowledge, this is the first study that evaluated the clinical significance of paxillin and EMMPRIN proteins in HCC patients.
Paxillin acts as an adaptor molecule in integrin signaling. Tyrosine phosphorylation of paxillin is a prominent event on integrin activation in normal epithelial cells[30-32]. Similarly, paxillin protein might be detected in normal (non-tumor) liver tissues. Although the percentage of adjacent non-tumor liver tissues that were positive for paxillin protein was higher than that of HCC, no significant difference was found between those two percentages. This finding suggested that paxillin protein existed in both the non-tumor liver cells and HCC cells, and interfered in their activities. Both adjacent non-tumor liver tissues and HCC tissues have the same chance to encounter carcinogenic factors and oncogenes. The paxillin-binding protein might attribute to the presence of paxillin protein in both normal (non-tumor) liver tissues and HCC. Furthermore, paxillin protein expression was associated with low differentiation, in the presence of portal vein thrombosis, along with extra-hepatic metastasis. Low differentiation, presence of portal vein thrombosis and extra-hepatic metastasis means high malignancy in HCC. So positivity of paxillin protein was related to the malignancy of HCC. Previous studies showed that protein kinase C (PKC) activator and 12-O-tetradecanoylphorbol-13-acetate (TPA) increased the expression of tyrosine phosphorylation of several proteins including the FAK and paxillin, which increased the invasion ability of human hepatocellular carcinoma cells in vitro[33-35]. However, paxillin protein was not related to the disease-free survival and overall survival in HCC patients. This finding suggested that detection of paxillin in HCC probably had little prognostic value, though this protein was related to the malignancy of HCC. Moreover, paxillin protein was not related to serum AFP level, HBsAg status, presence of microsatellite nodule, tumor size, and the presence of cirrhosis and necrosis.
Previous study has demonstrated that syndecan-1 expressed in normal epithelial cells[36]. Syndecan-1 protein was also detected in the adjacent non-tumor liver tissues[37]. This study showed that syndecan-1 exhibited positive expression in HCC. Interestingly, the percentage of syndecan-1 protein that were positive in both HCC and the adjacent non-tumor liver tissues was the same, and no significant difference was found between the percentages of those two kinds of tissues. Previous study showed that the expression of syndecan-1 reduced in HCC with intra-hepatic or extra-hepatic metastasis[37]. Similarly, this study had the same finding, in which there was a higher percentage of well differentiated HCC or HCC without extra-hepatic metastasis. This finding suggested that syndecan-1 protein was conversely related to the malignancy of HCC. Previous study had demonstrated that syndecan-1 was reduced both on the level of mRNA and protein levels in other cancers, and the reduction altered in different carcinomas[36]. Previously, it had been revealed that the expression of syndecan-1 was reduced in human hepatocellular carcinomas with high metastatic potential and speculated that syndecan-1 played an important role in inhibition of invasion and metastasis[26,36,37]. However, syndecan-1 protein was not related to the disease-free survival and overall survival in HCC patients, which suggested that it probably had little prognostic value in HCC. Furthermore, syndecan-1 protein was not related to serum AFP level, HBsAg status, the presence of microsatellite nodule, tumor size, presence of cirrhosis and necrosis, and the presence of portal vein thrombosis.
Matrix metalloproteinases (MMPs) play critical roles in the process of carcinogenesis, carcinoma invasion and metastasis by way of the proteolytic destruction of ECM and basement membranes[38]. There is a positive correlation between MMPs expression and the invasive and metastatic potential of malignant tumors, including colorectal, lung, prostate, bladder, HCC, pancreatic carcinomas, astrocytic and oligodendroglial gliomas[39-45]. EMMPRIN is a heavily glycosylated transmembrane glycoprotein containing two immunoglobulin superfamily domains, which induces MMPs production in the adjacent stromal cells. Some results implied that an EMMPRIN counter-receptor might have existed on the fiber cell surface, but such a counter-receptor has not been identified. This study showed that EMMPRIN protein was detected in the adjacent non-tumor liver tissues frequently, which was contrary to the finding of low expression in normal liver tissues. The percentage of EMMPRIN protein expression in HCC was higher than that in the adjacent non-tumor liver tissues. However, no significant reduction of EMMPRIN protein was found in adjacent non-tumor liver tissues compared with that in HCC. Other studies showed that EMMPRIN also acted in an autocrine fashion to increase productions of MMPs and invasiveness in tumor cells themselves. Previous studies demonstrated that EMMPRIN enriched in HCC tissue and these might be a potential target for anti-invasion and metastasis therapies. It was also shown that HAb18G/CD147 was highly expressed in HCC tissues and lowly expressed in normal tissues. HAb18G/CD147, not only participated in adhesion of cell-cell or cell-matrix but also enhanced metastatic potentials of human hepatoma cells by disrupting the regulation of store-operated Ca(2+) entry by NO/cGMP[13,14]. This study showed that EMMPRIN protein might express in HCC. However, EMMPRIN protein was not related to differentiation, presence of portal vein thrombosis and extra-hepatic metastasis. These findings suggested that the expression of EMMPRIN protein was not associated with the malignancy of HCC, which was contrary to the findings of MMPs expression[25]. Though those MMPs played an important role in the degradation of extracellular matrix of HCC, tissue inhibitor of matrix metalloproteinase proteins (TIMPs) also played an important role that inhibited the activities of MMPs in the degradation of extracellular matrix[25]. Moreover, this study showed that EMMPRIN was not related to the differentiation, presence of portal vein thrombosis and extra-metastasis. This was probably attributable to the finding that EMMPRIN protein was not related to the malignancy of HCC and the prognosis of HCC patients. Moreover, this study showed that patients with negative expression of EMMPRIN protein had better disease-free survival than those with positive expression, but the difference was not statistically significant. This was probably attributable to that EMMPRIN protein was only the inducer of MMPs, and the activity of MMPs was regulated by many factors such as TIMPs. This probably made the association of EMMPRIN protein with biological behaviors of HCC complicated. This study also showed that EMMPRIN might be detected in the HCC patients whose AFP serum levels were >25 ng/mL. And no significant difference of the expression of EMMPRIN protein was found between the patients with AFP serum levels >25 ng/mL and with those ≤25 ng/mL. This finding was not in agreement with the previous study. Moreover, EMMPRIN protein was not related to HBsAg status, presence of microsatellite nodule, tumor size and presence of cirrhosis and necrosis.
This study demonstrated that the expression of paxillin protein correlated with the expression of syndecan-1 protein conversely, which suggested that the two proteins played a contrary role in the progression and metastasis of HCC. Paxillin protein increased and syndecan-1 protein decreased the malignancy of HCC.
In conclusion, our data demonstrated that the expression of paxillin protein correlated with differentiation, the presence of portal vein thrombosis and extra-hepatic metastasis in HCC. Furthermore, the expression of syndecan-1 protein correlated with differentiation and extra-hepatic metastasis in HCC. Hence, both paxillin and syndecan-1 correlated with the malignancy of HCC, and those proteins played a contrary role in the progression and metastasis of HCC. Though EMMPRIN protein might express in HCC, no association was found between this protein and the malignancy of HCC.
Footnotes
Supported by the Major Programs of Health Bureau of Guangdong Province, No. A200194
Co-first-authors: De-Rong Xie
Edited by Li WZ Language Editor Elsevier HK
References
- 1.Srivatanakul P, Sriplung H, Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev. 2004;5:118–125. [PubMed] [Google Scholar]
- 2.Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8–24. [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Kiyosawa K, Tanaka E. Characteristics of hepatocellular carcinoma in Japan. Oncology. 2002;62 Suppl 1:5–7. doi: 10.1159/000048269. [DOI] [PubMed] [Google Scholar]
- 5.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Li L, Lu F. Mortality of primary liver cancer in China from 1990 through 1992. Zhonghua ZhongLiu ZaZhi. 1999;21:245–249. [PubMed] [Google Scholar]
- 7.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459–462. doi: 10.1136/gut.51.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe T, Omori M, Fukuda H, Takada H, Miyao M, Mizuno Y, Ohsawa I, Sato Y, Hasegawa T. Analysis of sex, age and disease factors contributing to prolonged life expectancy at birth, in cases of malignant neoplasms in Japan. J Epidemiol. 2003;13:169–175. doi: 10.2188/jea.13.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziparo V, Balducci G, Lucandri G, Mercantini P, Di Giacomo G, Fernandes E. Indications and results of resection for hepatocellular carcinoma. Eur J Surg Oncol. 2002;28:723–728. doi: 10.1053/ejso.2002.1299. [DOI] [PubMed] [Google Scholar]
- 11.Kanematsu T, Furui J, Yanaga K, Okudaira S, Shimada M, Shirabe K. A 16-year experience in performing hepatic resection in 303 patients with hepatocellular carcinoma: 1985-2000. Surgery. 2002;131:S153–S158. doi: 10.1067/msy.2002.119497. [DOI] [PubMed] [Google Scholar]
- 12.Aguayo A, Patt YZ. Liver cancer. Clin Liver Dis. 2001;5:479–507. doi: 10.1016/s1089-3261(05)70175-6. [DOI] [PubMed] [Google Scholar]
- 13.Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanova LY, Hashimoto S, Chay KO, Blagosklonny MV, Sabe H, Mushinski JF. Phosphorylation of paxillin tyrosines 31 and 118 controls polarization and motility of lymphoid cells and is PMA-sensitive. J Cell Sci. 2004;117:3759–3768. doi: 10.1242/jcs.01206. [DOI] [PubMed] [Google Scholar]
- 15.Sattler M, Pisick E, Morrison PT, Salgia R. Role of the cytoskeletal protein paxillin in oncogenesis. Crit Rev Oncog. 2000;11:63–76. [PubMed] [Google Scholar]
- 16.Turner CE. Paxillin interactions. J Cell Sci. 2000;113 Pt 23:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 17.Della Morte R, Squillacioti C, Garbi C, Derkinderen P, Belisario MA, Girault JA, Di Natale P, Nitsch L, Staiano N. Echistatin inhibits pp125FAK autophosphorylation, paxillin phosphorylation and pp125FAK-paxillin interaction in fibronectin-adherent melanoma cells. Eur J Biochem. 2000;267:5047–5054. doi: 10.1046/j.1432-1327.2000.01561.x. [DOI] [PubMed] [Google Scholar]
- 18.Roskams T, De Vos R, David G, Van Damme B, Desmet V. Heparan sulphate proteoglycan expression in human primary liver tumours. J Pathol. 1998;185:290–297. doi: 10.1002/(SICI)1096-9896(199807)185:3<290::AID-PATH91>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Kiviniemi J, Kallajoki M, Kujala I, Matikainen MT, Alanen K, Jalkanen M, Salmivirta M. Altered expression of syndecan-1 in prostate cancer. APMIS. 2004;112:89–97. doi: 10.1111/j.1600-0463.2004.apm1120202.x. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu T, Miyauchi T. Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol. 2003;18:981–987. doi: 10.14670/HH-18.981. [DOI] [PubMed] [Google Scholar]
- 21.Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, Zucker S, Toole BP. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004;64:1229–1232. doi: 10.1158/0008-5472.can-03-2832. [DOI] [PubMed] [Google Scholar]
- 22.Dalberg K, Eriksson E, Enberg U, Kjellman M, Bäckdahl M. Gelatinase A, membrane type 1 matrix metalloproteinase, and extracellular matrix metalloproteinase inducer mRNA expression: correlation with invasive growth of breast cancer. World J Surg. 2000;24:334–340. doi: 10.1007/s002689910053. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–2281. [PubMed] [Google Scholar]
- 24.Hefler LA, Concin N, Mincham D, Thompson J, Swarte NB, van Eijkeren MA, Sie-Go DM, Hammond I, McCartney AJ, Tempfer CB, et al. The prognostic value of immunohistochemically detected CD44v3 and CD44v6 expression in patients with surgically staged vulvar carcinoma: a multicenter study. Cancer. 2002;94:125–130. doi: 10.1002/cncr.10206. [DOI] [PubMed] [Google Scholar]
- 25.Wei QY, Wu YQ, Fan SQ. Expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in the hepatocellular carcinomas. Hunan YiKe DaXue XueBao. 2003;28:212–216. [PubMed] [Google Scholar]
- 26.Ohtake T, Fujimoto Y, Ikuta K, Saito H, Ohhira M, Ono M, Kohgo Y. Proline-rich antimicrobial peptide, PR-39 gene transduction altered invasive activity and actin structure in human hepatocellular carcinoma cells. Br J Cancer. 1999;81:393–403. doi: 10.1038/sj.bjc.6690707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyton J, Garcia-Marin LJ, Tapia JA, Jensen RT, Moody TW. Bombesin and gastrin releasing peptide increase tyrosine phosphorylation of focal adhesion kinase and paxillin in non-small cell lung cancer cells. Cancer Lett. 2001;162:87–95. doi: 10.1016/s0304-3835(00)00639-x. [DOI] [PubMed] [Google Scholar]
- 28.Mennerich D, Vogel A, Klaman I, Dahl E, Lichtner RB, Rosenthal A, Pohlenz HD, Thierauch KH, Sommer A. Shift of syndecan-1 expression from epithelial to stromal cells during progression of solid tumours. Eur J Cancer. 2004;40:1373–1382. doi: 10.1016/j.ejca.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Han JL, Xie WL, Huang J, Yao YS. Expression of extracellular matrix metalloproteinase inducer in human bladder transitional cell carcinoma. AiZheng. 2003;22:1158–1161. [PubMed] [Google Scholar]
- 30.Yano H, Uchida H, Iwasaki T, Mukai M, Akedo H, Nakamura K, Hashimoto S, Sabe H. Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc Natl Acad Sci USA. 2000;97:9076–9081. doi: 10.1073/pnas.97.16.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu CF, Sanders MA, Basson MD. Human caco-2 motility redistributes FAK and paxillin and activates p38 MAPK in a matrix-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2000;278:G952–G966. doi: 10.1152/ajpgi.2000.278.6.G952. [DOI] [PubMed] [Google Scholar]
- 32.Umino T, Wang H, Zhu Y, Liu X, Manouilova LS, Spurzem JR, Patricia Leuschen M, Rennard SI. Modification of type I collagenous gels by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2000;22:702–707. doi: 10.1165/ajrcmb.22.6.3806. [DOI] [PubMed] [Google Scholar]
- 33.Imamura F, Mukai M, Ayaki M, Akedo H. Y-27632, an inhibitor of rho-associated protein kinase, suppresses tumor cell invasion via regulation of focal adhesion and focal adhesion kinase. Jpn J Cancer Res. 2000;91:811–816. doi: 10.1111/j.1349-7006.2000.tb01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu LC, Chou CK, Chen HC, Yeh SF. Protein kinase C-mediated tyrosine phosphorylation of paxillin and focal adhesion kinase requires cytoskeletal integrity and is uncoupled to mitogen-activated protein kinase activation in human hepatoma cells. J Biomed Sci. 2001;8:184–190. doi: 10.1007/BF02256411. [DOI] [PubMed] [Google Scholar]
- 35.Yoon WH, Song IS, Lee BH, Jung YJ, Kim TD, Li G, Lee TG, Park HD, Lim K, Hwang BD. Differential regulation of vimentin mRNA by 12-O-tetradecanoylphorbol 13-acetate and all-trans-retinoic acid correlates with motility of Hep 3B human hepatocellular carcinoma cells. Cancer Lett. 2004;203:99–105. doi: 10.1016/j.canlet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Watari J, Saitoh Y, Fujiya M, Shibata N, Tanabe H, Inaba Y, Okamoto K, Maemoto A, Ohta T, Yasuda A, et al. Reduction of syndecan-1 expression in differentiated type early gastric cancer and background mucosa with gastric cellular phenotype. J Gastroenterol. 2004;39:104–112. doi: 10.1007/s00535-003-1260-2. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto A, Ono M, Fujimoto Y, Gallo RL, Bernfield M, Kohgo Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. Int J Cancer. 1997;74:482–491. doi: 10.1002/(sici)1097-0215(19971021)74:5<482::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Freije JM, Balbín M, Pendás AM, Sánchez LM, Puente XS, López-Otín C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 39.Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int J Cancer. 2003;107:541–550. doi: 10.1002/ijc.11436. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Sakurai H, Oshima K, Kim SH, Saiki I. Anti-metastatic and anti-angiogenic activities of a new matrix metalloproteinase inhibitor, TN-6b. Eur J Cancer. 2003;39:1632–1641. doi: 10.1016/s0959-8049(03)00375-7. [DOI] [PubMed] [Google Scholar]
- 41.London CA, Sekhon HS, Arora V, Stein DA, Iversen PL, Devi GR. A novel antisense inhibitor of MMP-9 attenuates angiogenesis, human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther. 2003;10:823–832. doi: 10.1038/sj.cgt.7700642. [DOI] [PubMed] [Google Scholar]
- 42.Gakiopoulou H, Nakopoulou L, Siatelis A, Mavrommatis I, Panayotopoulou EG, Tsirmpa I, Stravodimos C, Giannopoulos A. Tissue inhibitor of metalloproteinase-2 as a multifunctional molecule of which the expression is associated with adverse prognosis of patients with urothelial bladder carcinomas. Clin Cancer Res. 2003;9:5573–5581. [PubMed] [Google Scholar]
- 43.Ishii Y, Nakasato Y, Kobayashi S, Yamazaki Y, Aoki T. A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J Exp Clin Cancer Res. 2003;22:461–470. [PubMed] [Google Scholar]
- 44.Harvey SR, Hurd TC, Markus G, Martinick MI, Penetrante RM, Tan D, Venkataraman P, DeSouza N, Sait SN, Driscoll DL, et al. Evaluation of urinary plasminogen activator, its receptor, matrix metalloproteinase-9, and von Willebrand factor in pancreatic cancer. Clin Cancer Res. 2003;9:4935–4943. [PubMed] [Google Scholar]
- 45.Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res. 2003;23:3937–3944. [PubMed] [Google Scholar]


