Abstract
AIM: To transfer human HGF gene into the liver of rats by direct electroporation as a means to prevent radiation-induced liver damage.
METHODS: Rat whole liver irradiation model was accomplished by intra-operative approach. HGF plasmid was injected into liver and transferred by electroporation using a pulse generator. Control rats (n = 8) received electrogene therapy (EGT) vehicle plasmid and another 8 rats received HGF-EGT 100 μg 48 h before WLIR. Expression of HGF in liver was examined by RT-PCR and ELISA methods. Apoptosis was determined by TUNEL assay. Histopathology was evaluated 10 wk after whole liver irradiation.
RESULTS: Marked decrease of apoptotic cells and down-regulation of transforming growth factor-beta 1 (TGF-β1) mRNA were observed in the HGF-EGT group 2 d after liver irradiation compared to control animals. Less evidence of radiation-induced liver damage was observed morphologically in liver specimen 10 wk after liver irradiation and longer median survival time was observed from HGF-EGT group (14 wk) compared to control rats (5 wk). (P = 0.031).
CONCLUSION: For the first time it has been demonstrated that HGF-EGT would prevent liver from radiation-induced liver damage by preventing apoptosis and down-regulation of TGF-β1.
Keywords: Hepatocyte growth factor, HGF, Radiation, Liver, Electroporation, Electrogene therapy
INTRODUCTION
Radiation-induced liver damage is the end stage of acute liver injury. The damage caused by radiation is characterized by the development of anicteric ascites approximately 2 wk to 4 mo after hepatic irradiation. Radiotherapy traditionally had a limited role in the treatment of unresectable primary or metastatic intrahepatic cancers primarily due to the low liver tolerance to radiotherapy. Radiation-induced liver damage had been observed in 5-10% of patients who had received whole liver radiation dose exceeding 30 Gy[1]. Although 3-D conformal radiotherapy may greatly reduce the normal liver irradiated volume through dose volume histogram evaluation, the radiation-induced liver damage is the dose-limiting factor for liver tumor irradiation. Recently developed intra-arterial radiolabeled-iododeoxyuridine therapy, lipiodol-131I therapy, or boron neutron capture therapy for liver cancers are all also potentially limited by the risk of liver damage[2-4]. The major pathological feature of radiation-induced liver damage is veno-occlusive disease. The biological foundations of radiation-induced liver damage are poorly understood. The standard treatment is limited and has a high mortality rate.
HGF is a pluripotent factor associated with tissue growth and regeneration[5]. HGF is a strong promoter of cell survival[6,7]. It promotes the differentiation of mesenchymal cells to epithelial or endothelial cells and plays a pivotal role in the prevention of tissue fibrosis and dysfunction[8-10]. Moreover, HGF is considered to be a major determinant of whether the epithelium remains in a quiescent state or shifts to a proliferative state associated with development and tissue repair[8,11].
Under normal conditions, liver, kidney and spleen produce low amounts of HGF. Noticeable pathological changes such as ischemia, toxic injury or tissue loss would result in the increase of HGF and its receptor c-Met during the early stage of injury[12]. Although endogenous HGF levels are elevated after tissue injury, the amounts are low during the damage occurrence. Furthermore, high and sustained amounts of HGF are mandatory for subsequent tissue repair. Therefore, the use of exogenous HGF therapy may be an effective treatment for the prevention of tissue injury and the promotion of tissue repair[13]. Recombinant HGF has a short half life of 3-5 min, local regional continuous infusion by intra-arterial pump would be an appropriate delivery method to achieve high and sustained amount of HGF to liver[12]. In addition, an alternative method for sustained delivery of pharmacological level of protein in target tissue would be based upon a gene therapy-mediated approach.
Researchers reported that intra-muscular (IM) injection of HGF genes ameliorated chemically-induced liver damage in rabbits[14]. In vivo electrogene transfer (EGT) is considered a highly efficient gene transfer method[15]. Electricity-mediated HGF gene transfer into muscle has also been reported to accelerate regeneration in cirrhotic livers of mice after partial hepatectomy[9]. However, there was no report of HGF gene therapy on the protection of radiation damage. The present study was designed to assess the efficacy of direct EGT with HGF gene into livers of rats as a method to prevent radiation-induced liver damage.
MATERIALS AND METHODS
Plasmid DNA
Plasmid PCR3.1/hHGF was constructed by inserting the full length cDNA of human HGF under a human cytomegalovirus promoter. Human HGF cDNA (2.2 kb) was subcloned into two KpnI sites. Plasmids were grown in TOP10F’ competent cell, selected by ampicillin and extracted by an EndoFree Plasmid Giga kit (Qiagen, Valencia, CA). Enhanced green fluorescent protein (pIRES2-EGFP) plasmid was obtained commercially (Clontech, Palo Alto, CA, USA). DNA was dissolved in EndoFree TE buffer, and the quality and quantity were assessed by measuring the optical density at 260 and 280 nm.
Liver irradiation
Sprague-Dawley (SD) female rats (180-200 g) received whole liver radiation. The experiment was approved by Animal Committee of Veterans General Hospital-Taipei. Three dosage levels were chosen and each dosage level included eight animals. The measured variables were body weight change, serum aspartate aminotransferase (AST), alkaline aminotransferase (ALT) changes. An autopsy was performed with specific attention to the liver histopathology and animal survival proportion was counted. Before irradiation, each animal was anesthetized with ketamine, and its abdomen was opened. Most of the intestine, stomach, and spleen were not within the radiation field. All organs were kept moist with gauze soaked in lactate Ringer’s solution during irradiation of 20 Gy, 40 Gy and 65 Gy with a Cobalt machine. The rats were warmed with a light heater to prevent hypothermia. The animal’s muscle layer was sewn together with sterile 3-0 nylon sutures after irradiation. The skin was closed with surgical clips.
EGT-HGF plasmids into liver
In order to observe the HGF protein and mRNA expression after direct HGF gene transfection by electroporation, we investigated dose-responsive relationship of EGT HGF. The left median or left lateral lobe of the liver was exposed after the abdominal cavity was opened surgically. The center of the lobe was caught between the tweezer-type electrode disks. PCR3.1/hHGF plasmid DNA in 0, 25, 50, 100, 200 μg/100 μ/L volume was injected with a microinjector into the liver halfway between the two electrode disks. Immediately after the DNA injection, electric pulses were administered. The electrical pulses were delivered using an Electro Square porator (T820, BTX, San Diego, CA)[16,17] The rat’s liver was electroporated with 8 electrical pulses of 50 ms duration at 50 V. One to eight electric pulses were administered at a rate of one pulse per second. The abdominal wound was then closed. The efficacy of liver EGT was checked with 50 μg EGFP plasmid co-administered with HGF gene 50 μg under fluorescence microscopy. Protein extracts from the liver were prepared 2 d after electroporation. The concentrations of HGF in rat liver extracts after different doses of EGT-HGF were determined by enzyme-linked immunoassay (ELISA) kit for human HGF. (R & D Systems, Inc., Minneapolis, Minnesota, USA).
Therapeutic effect of EGT-HGF for the prevention of radiation-induced liver damage
The liver of each rat received EGT 48 h prior to a liver irradiation of 65 Gy. The experimental groups (10 rats) were transfected with PCR3.1/hHGF plasmid 100 g/liver, and the control group (10 rats) was electroporated with empty vector 100 g/liver. Blood samples were collected weekly for 10 wk to examine the serial AST/ALT and the changes in the transforming growth factor (TGF-β1). One rat from each group was sacrificed 2 d after irradiation to examine the apoptosis formation and TGF-β1 expression in its liver sections. Serum HGF concentrations were determined 7 d after EGT. One rat from each group was sacrificed 10 wk after irradiation to investigate the histopathological changes. The overall survival time was recorded.
Identification of apoptosis
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeled (TUNEL) assay was performed for the detection of apoptotic cells. Apoptotic hepatocytes were labeled in situ using a FragELTM DNA fragmentation detection kit-TdT enzyme (Oncogene, Boston, MA) according to the manufacturer’s stipulations. First, 3 μm thick deparaffinized liver sections were pretreated with proteinase K (20 μg/mL) for 20 min at room temperature. After washing in TBS, the endogenous peroxidase was inactivated in 3% H2O2 for 5 min, followed by incubation with TdT labeling reaction mixture at 37 °C for 1.5 h in a humid chamber. After this incubation, sections were incubated with stop solution for 5 min, blocking buffer for 10 min, and 1×conjugate buffer for 30 min. Finally, sections were incubated with diaminobenzidine (DAB) solution for 10-15 min, followed by incubation with methyl green as counterstain. Apoptotic cells were counted using a light microscope at a magnification of ×100. Image quantification was performed using Image-Pro Plus 4.1 program from Media Cybernetics (Silver Spring, MD) to determine the percent apoptotic cells. In addition, some paraffin-embedded liver sections were stained to assess histopathological changes.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total cellular RNA was isolated from snap-frozen liver tissue by using RNAzolTM B (Tel-Test, Friendswood, TX) according to the manufacturer’s instruction. 1-3 μg of RNA was converted to complementary DNA by using SuperscriptTMII Rnase H Reverse Transcriptase (Invitrogen, Carlsbad, CA). Primer sequences for human HGF, TGF-β1, and β-globin, used in PCR on complementary DNA in each reaction were shown in Table 1. After the complementary DNA was amplified by using primer HGF-1 and TGF-β1-1, the PCR product was amplified a second time by using primer HGF-2 and TGF-μ1-2. The cycle number of PCR was 30 for all genes.
Table 1.
Primers for PCR.
| Gene | Primer sequences |
| HGF-1 | Sense: 5-CCAGCAGCACCATGTGGG-3’ |
| Antisense: 5-CAGCTATGACTGTGGTACC-3’ | |
| HGF-2 | Sense: 5-CTGCTGCTGCAGCATGTCC-3’ |
| Antisense: 5-GGCACATCCACGACCAGG-3’ | |
| TGFβ | Sense: 5-GCTGCTGCCGCTTCTGCTCC-3’ |
| Antisense: 5-GCACTTGCAGGAGCGCACG-3’ | |
| TGF-β1-2 | Sense: 5-GGCTTCTAGTGCTGACGCCCG-3’ |
| Antisense: 5-CCACCTTGGGCTTGCGACCC-3’ | |
| β-globin | Sense: 5-CCAATCTGCTCACACAGGATAGAGAGGGCAGG-3’ |
| Antisense: 5-CCTTGAGGCTGTCCAAGTGATTCAGGCCATCG-3’ |
Histocytochemistry
Autopsies were performed. The liver and adjacent organs were visually examined to determine the possible cause of death. One rat from each group was sacrificed on wk 10. The liver was removed and fixed in buffered formalin and stained with Masson trichrome staining method. Deparaffinized sections were hydrated with distilled water. Each section was rinsed well in distilled water and stained step by step with Weigert’s hematoxylin for 10 min. This was followed by a Biebrich scarlet-acid fuchsin solution for 15 min. Then the sample was treated with phosphomolybdic-phosphotungstic acid for 15 min. The next phase was to rinse with aniline blue solution for 10 min at RT. The final process involved differentiation for 2 min in a 1% acetic acid, dehydration and finally mounted with synthetic resin.
Statistical analysis
Results are expressed as mean±SD. Statistical difference was assessed by the unpaired two-tailed Student’s t test. The survival data was determined by Kaplan-Meier method and a log-rank test was performed to compare the difference. P<0.05 was considered significant.
RESULTS
Radiation-induced liver damage model
Single fraction radiation of 20-65 Gy to liver was performed intra-operatively with Cobalt machine. Rats that received 40 Gy and 65 Gy of irradiation gradually had a decreased body weight and activity. There was no significant weight reduction in the rats that received 20 Gy irradiation. All the groups were observed for three months after radiation. None of the rats in the 20 Gy group died of liver irradiation. Fifty percent of the rats survived after 40 Gy, and only twenty-five percent of the rats survived after 65 Gy of liver irradiation at wk 12. All rats were autopsied and each showed evidence of jaundice as indicated by yellow coloration of the skin, urine and plasma. Ascites and sometimes pleural effusion were also present. No irradiation-related changes of adjacent intra-abdominal organs were seen by macroscopic inspection. The liver surface was adhered to the parietal peritoneum forming dense fibrous tissue. Early deaths (≤6 wk) were associated with hepatic congestion and swelling. Late deaths (≥10 wk) were usually associated with a shrunken and sometimes deformed liver, which indicated fibrosis formation. Microscopically, there were distortion of liver cellular architecture, sinusoid congestion, interstitial fibrosis, inflammatory cell infiltrations, bile duct proliferation and focal hepatocyte necrosis around hepatic veins. The pathology of 20 Gy irradiated animals was not noticeably different from normal control except for the varying congestion and dilatation of sinusoids and slight infiltration of inflammatory cells. There was no significant change of AST/ALT level in all treatment groups. However, the transient elevation of the ALT levels were observed in the second wk in the 65 Gy group, 6th wk in the 40 Gy group and 10th wk in the 20 Gy group. Brown apoptotic hepatocytes stained with TUNEL assay were observed 2 d after radiation and were also visible 2 wk later (data not shown).
Expression of HGF protein and mRNA after direct liver EGT with HGF plasmid
The liver of rats, which had 65 Gy of irradiation were used in the HGF-EGT experiments. PCR3.1/hHGF plasmid DNA in 0, 25, 50, 100, and 200 μg/100 μL were electroporated into the rat’s liver and killed them 48 h later. Figure 1 indicated a dose-dependent increase of HGF concentrations in liver protein extracts by ELISA. The HGF expression reached a plateau after 100 μg of plasmid transfection by EGT. Green fluorescent protein can be observed in the cytoplasm of hepatocytes from double-gene transfection groups. Green fluorescence protein expressions were detected within 24 h, maximally at 3 d, and persisted for 3 wk but gradually reduced in intensity. The estimated transfection efficiency was around 40% 48 h after EGFP electroporation as assessed by fluorescence intensity under microscopic images examination (data not shown). RT-PCR disclosed human HGF segment of 2.2 kb in length 2 d after the EGT (data not shown).
Figure 1.
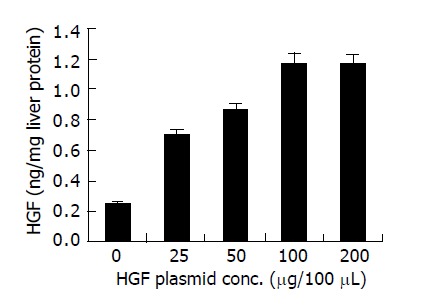
ELISA of human HGF protein concentrations in extracts of liver tissue taken from electroporated area 2 d after EGT-HGF.
Therapeutic effect of liver in situ EGT-HGF for the prevention of radiation-induced liver damage
Two days after 65 Gy of irradiation, one rat from each group was killed for an apoptosis study. Overall, there was a mild swelling and a petechial change over the electroporated field. TUNEL staining revealed a significant increase in apoptotic cells in the liver specimen. As shown in Figure 2, pre-administration of HGF plasmid by EGT 48 h before radiation markedly prevented formation of apoptotic cells in the liver. Most apoptosis occurred around the microvascular structures. Ten week after irradiation, one rat from each group was sacrificed for histological study. Grossly, moderate amount of ascites, jaundice, pleural effusion and a shrunken liver were observed in the control group. The degree of gross abnormality was less in the EGT-HGF group. Microscopically, congested hepatic veins, marked proliferation of bile ducts, increased fibrosis and collagen around hepatic veins, concentric lamellations and subintimal thickening of hepatic arteries were observed in liver sections stained with Masson trichrome method (Figure 3). There were fewer fibrosis in the rats treated with EGT-HGF than radiation alone group. Biochemically, the general patterns of serum ALT/AST showed no significant difference between two groups except for the transient elevation of ALT level one wk after irradiation in the control group (mean 43.6±35.8 vs 53.4±30.8, P = 0.2, Figure 4). EGT-HGF treatment seemed to suppress the initial elevation of ALT level. The relative insensitivity of ALT/AST changes for monitoring radiation-induced liver damage indicated that liver parenchymal cell necrosis was not the major pathological change from radiation damage. The serum TGF-β1 was also measured without consistent patterns for reliable prediction of liver damage (data not shown). No serum human HGF was detected by the ELISA kit one wk after the administration of EGT. Overproduction of TGF-β1 is a major cause of radiation-induced tissue fibrosis in liver and TGF-β1 induces apoptotic cell death in hepatocytes. The expression of TGF-β1 in liver 48 h after radiation from both groups by RT-PCR was investigated. TGF-β1 expression was markedly induced in the RT alone group while the TGF-β1 expression was down-regulated in rats treated with EGT-HGF (Figure 5). Rats, which received whole liver irradiation began to die 4 wk after irradiation. EGT-HGF treatment had prolonged the median survival time from 5 wk to 14 wk. By the end of 24 wk, the overall survival was 0% in the control group and 37.5% in the EGT-HGF group (Figure 6) (P = 0.031, Log-rank test).
Figure 2.
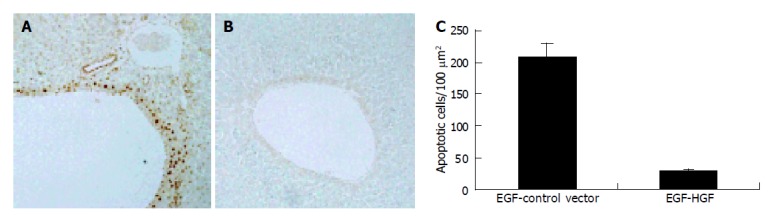
Apoptotic activities (TUNEL analysis) in rat liver after 65 Gy of irradiation. A: EGT-control vector 2 d before liver irradiation; B: EGT-HGF 2 d before irradiation. Rats were sacrificed 2 d after irradiation and stained with TUNEL assay. Note the brown round stain indicated TUNEL-positive cells, which gathered around microvascular region. EGT-HGF almost completely protected rat liver from radiation-induced apoptosis; C: Percent of apoptotic cells were quantitated per 100 μm2 in areas shown on A and B. (P<0.05).
Figure 3.
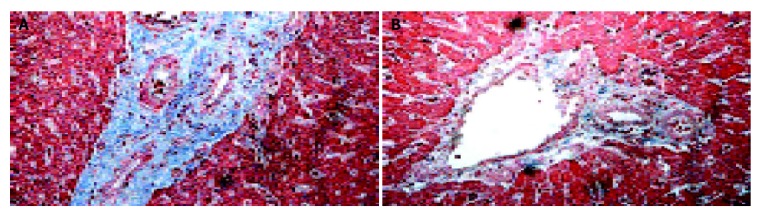
Histopathology of the liver 10 wk after 65 Gy of irradiation. A: rat liver (×200) receiving EGT-control-vector followed by 65 Gy of liver radiation 48 h later; B: rat liver (×200) receiving EGT-HGF followed by 65 Gy of liver radiation 48 h later. The blue stain indicated fibrosis formation. Fibrosis is more extensive with evidence of bile duct proliferation, sub-intimal thickening of arterioles with compromise of the vessel lumens. The morphology changes were less severe in HGF gene therapy group.
Figure 4.
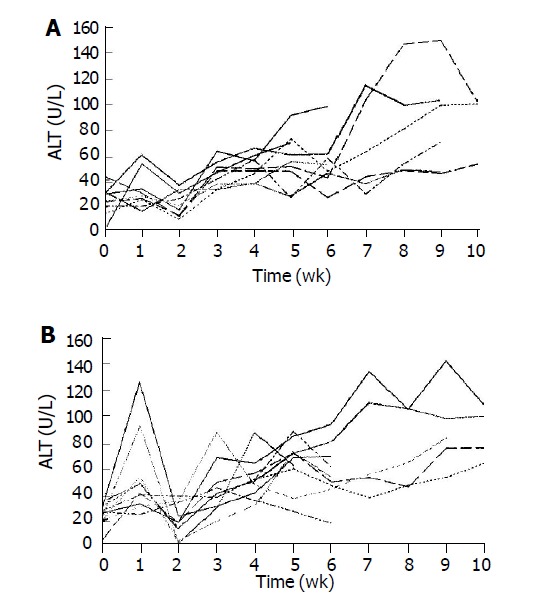
Weekly plasma ALT changes in rats receiving whole liver irradiation. A: Serial plasma ALT changes in EGT-HGF plus radiation group; B: Serial plasma ALT changes in EGT-control vector plus radiation group.
Figure 5.
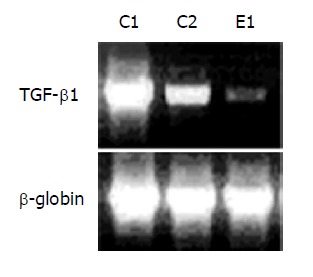
TGF-β1 RT-PCR analysis 2 d after liver irradiation. C1: rats receiving EGT-control vector and 65 Gy of liver irradiation; C2: rats receiving EGT-control vector and 20 Gy of liver irradiation; E1: rats receiving EGT-HGF and 65 Gy of liver irradiation. Figure is the representative of 3 experiments.
Figure 6.
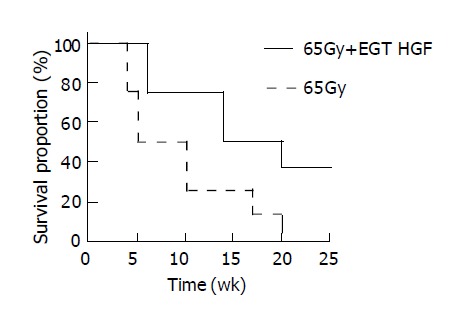
Survival analyses of rats after whole liver irradiation of 65 Gy. Segmented line (n = 8) indicated group that received EGT-control vector plus radiation treatment. Solid line (n = 8) indicated group that received EGT-HGF plus radiation treatment.
DISCUSSION
In this study, HGF plasmid gene transferred into liver by electroporation has been demonstrated to be effective in the prevention of RILD in the liver. The introduction of HGF gene by electroporation not only prevented the apoptosis from radiation damage, but also improved overall survival of the rats. Electroporation, a non-viral gene transfer method has been widely used to introduce DNA into various types of cells and organ[18]. The use of EGT in a liver had been regarded as one of the most efficient method of delivering and expressing high levels of exogenous genes in liver[19].
HGF gene therapy by EGT HGF plasmid had been successfully reported in prolonging survival time in chemically-induced liver cirrhosis with or without hepatectomy models[9,14,20]. The expression patterns of HGF transfer involving IM EGT is better than gene transfer using IM high-dose adenoviral vector as it is very difficult to sustain HGF levels due to adverse immune responses[21]. In case of tumors which are not as permissive to adenovirus, it is difficult to transduce a large number of tumor cells with adenoviral vectors without inducing severe liver damage. In contrast, the systemic toxicity is not a risk with plasmid electroporation as with adenoviral transfection. In this study, the liver electro-transfection efficiency of transferred gene expression was observed from the co-transfection EGFP plasmid by fluororescence microscopic image. Electroporation with eight electric pulses of 50 ms duration at 50 V gave a good efficiency of transfection as judged by the induced GFP expression. The transfection efficiency increased as the amount of injected DNA was increased but seemed to reach a plateau after 100 μg of plasmid per transfection. The volume and techniques of plasmid delivery limited the maximal dose that could be delivered into the liver by direct parenchyma injection. This technical obstacle may be solved by producing micro-encapsuled plasmid injected via portal vein in order to increase the plasmid retention in the liver parenchyma before electroporation. The feasibility of HGF gene transfer into the liver via the portal vein injection followed by liver parenchyma electroporation has been reported to attenuate rat liver cirrhosis[22].
In this experiment SD rat could tolerate high dose of whole liver irradiation with an LD50 dose around 50 Gy. Acute lethal intestinal injury was prevented by moving intestine and stomach out of irradiation field. The advantage of intra-operative whole liver irradiation is that one can confirm visually that the liver is in the irradiation field and most of the unnecessary irradiated gastrointestinal tract is out of the irradiation field. Decreased hepatic function, ascites, pleural effusion, jaundice, elevated AST/ALT and histologic changes such as necrosis, parenchymal cell loss, congested sinusoids, hemorrhagic extravasation, portal area fibrosis and collagen around hepatic veins with concentric lamellations and subintimal thickening of hepatic arteries, which were observed in the present study are similar to veno-occlusive in humans[23,24]. Gerace et al[25] had reported similar histological lesion with evidences of injury to hepatic veins after whole liver irradiation of rats. Hebard et al[26] had reported that the threshold radiation dose for vein damage was less than the threshold dose for direct cell killing of hepatocytes. AST/ALTs are parenchymal intracellular enzymes released into systemic circulation when there is hepatocellular injury and necrosis. Gerace et al[25] had reported an average of 3 times higher level of elevation of AST/ALT approximately 40 d after irradiation, which coincided with the clearance of rose bengal. Nevertheless, the results of this study indicated that both AST and ALT are not sensitive enough to detect radiation-induced liver damage through out the first 10 wk except some transient elevation of ALT serum levels one wk after 65 Gy of radiation. Apoptosis occurs early after irradiation, which may be a better indicator of radiation damage, yet it needs tissue biopsy. Serum TGF-β1 may be an alternatives for the early prediction of radiation induced injury. Murase et al[27] have demonstrated that an elevated plasma TGF-β1 concentration before transplant predicted the later occurrence of veno-occlusive disease complications. However, the present experiment failed to show correlation of TGF-β1 dynamic changes between groups with or without HGF after irradiation.
Under normal conditions, liver, kidney and spleen produce low amounts of HGF. Following damage such as ischemia, toxic injury or tissue loss, HGF and its tyrosine kinase receptor c-Met increase during early stage after injury[12]. HGF and c-Met, forming complex have been shown to interfere with apoptosis induced by ionizing radiation in vitro[28,29]. We found TUNEL-positive signals were reduced compared to those in the radiation alone group by pre-radiation EGT-HGF. This finding indicated that HGF suppresses radiation-induced apoptosis. The apoptotic cells mostly gather around the microvascular structures from TUNEL assay. Recent evidence suggests that microvascular endothelial apoptosis represents the primary lesion in radiation damage[30]. Endothelial injury of sinusoids and small hepatic veins is considered to be the initial event in genesis of veno-occlusive disease. Emerging data suggest that radiation acts directly on the plasma membrane of several cell types, activating acid sphingomyelinase, which generate ceramide by enzymatic hydrolysis of sphingomyelin. Ceramide, then, acts as a second messenger in initiating an apoptotic response via the mitochondrial system. Radiation-induced DNA damage can also initiate ceramide generation by activation of mitochondrial ceramide synthase and de novo synthesis of ceramide[30]. HGF acts to stabilize the cell membrane in capillary endothelial cells and may play the most important role in the prevention of radiation induced apoptosis.
In addition to the direct anti-apoptosis effect of HGF during radiation, professionals need to incorporate a therapeutic strategy focused on the promotion of liver regeneration and the prevention of subsequent fibrosis after radiation damage[5,31]. HGF is a well known hepatotropic factor for liver regeneration and has been reported to increase hepatic collagenase activity, which promotes degradation of the extracellular matrix components[5,31]. Another major benefit of HGF treatment after radiation is the reduction of TGF-β1 mRNA levels. TGF-β1 is a crucial factor in liver fibrosis and a potent growth inhibitor of hepatocytes. TGF-β1 is synthesized in non-parenchymal cells such as stellate cells and inhibits hepatocellular DNA synthesis both in culture and in vivo[32]. HGF and TGF-β1 are key molecules which in general, exert opposite actions[33]. Geraci et al[34] had reported the TGF-β1 mRNA markedly expressed in non-parenchymal cells of rat liver early after radiation and the expression may last for more than 90 d. The present research demonstrated that irradiation rapidly induced TGF-β1 mRNA expression and HGF gene transfer could antagonize the TGF-β1 expression early after irradiation. A sustained release of HGF (about 3 wk) from single EGT may provide crucial antagonistic effect on a persistent TGF-β1 expression after RT. In addition, fibronectin production from injured hepatic stellate cells was greatly reduced[35].
The incidence of radiation induced liver damage would be expected to increase due to a renewed interest in hepatic irradiation because of the introduction of three dimensional radiation therapy treatment planning and bone marrow transplantation using total body irradiation. The mortality rate of veno-occlusive disease is high ranged from 20 to 50% and is dose-limiting for liver radiotherapy. The data from this study suggests that HGF gene transfer by electroporation may inhibit radiation-induced apoptosis and down-regulate the elevated TGF-β1 levels after high dose irradiation for the prevention of veno-occlusive disease and liver fibrosis. Administration of HGF gene to normal liver tissue by electroporation during surgical procedure is clinically feasible, which may permit the safe delivery of high doses of radiation to an unresectable or residual hepatoma.
ACKNOWLEDGEMENTS
The work was supported by National Science Council grant NSC-91-275-9075-001 for the development of Boron Neutral Capture Therapy for hepatoma directed by Dr. Lui WY in Veterans General Hospital-Taipei.
Footnotes
Supported by National Science Council grant NSC-91-275-9075-001 for the development of Boron Neutron Capture Therapy for Hepatoma Treatment
Edited by Guo SY Language Editor Elsevier HK
References
- 1.Dawson LA, Ten Haken RK, Lawrence TS. Partial irradiation of the liver. Semin Radiat Oncol. 2001;11:240–246. doi: 10.1053/srao.2001.23485. [DOI] [PubMed] [Google Scholar]
- 2.Chi KH, Wang HE, Chen FD, Chao Y, Liu RS, Chou SL, Wang YS, Yen SH. Preclinical evaluation of locoregional delivery of radiolabeled iododeoxyuridine and thymidylate synthase inhibitor in a hepatoma model. J Nucl Med. 2001;42:345–351. [PubMed] [Google Scholar]
- 3.Lui WY, Liu RS, Chiang JH, Lo JG, Lai KH, King KL, Cheng HC, Wei YY, Chi CW, P'eng FK. Report of a pilot study of intra-arterial injection of I-131 lipiodol for the treatment of hepatoma. Zhonghua YiXue ZaZhi (Taipei) 1990;46:125–133. [PubMed] [Google Scholar]
- 4.Lin WY, Chi CW, Ho YJ, Wu IC, Chung YT, Chen SD, Chou FI, Kai JJ, Lui WY, Chen TJ, et al. Boron-lipiodol: a potential new drug for the treatment of liver tumors. Anticancer Res. 2002;22:3989–3992. [PubMed] [Google Scholar]
- 5.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, DeFrances MC, Dai Y, Pediaditakis P, Johnson C, Bell A, Michalopoulos GK, Zarnegar R. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell. 2002;9:411–421. doi: 10.1016/s1097-2765(02)00439-2. [DOI] [PubMed] [Google Scholar]
- 7.Derksen PW, de Gorter DJ, Meijer HP, Bende RJ, van Dijk M, Lokhorst HM, Bloem AC, Spaargaren M, Pals ST. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia. 2003;17:764–774. doi: 10.1038/sj.leu.2402875. [DOI] [PubMed] [Google Scholar]
- 8.Kopp JB. Hepatocyte growth factor: mesenchymal signal for epithelial homeostasis. Kidney Int. 1998;54:1392–1393. doi: 10.1046/j.1523-1755.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- 9.Xue F, Takahara T, Yata Y, Kuwabara Y, Shinno E, Nonome K, Minemura M, Takahara S, Li X, Yamato E, et al. Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy. Gut. 2003;52:694–700. doi: 10.1136/gut.52.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto J, Kaneda Y. Reversing liver cirrhosis: impact of gene therapy for liver cirrhosis. Gene Ther. 1999;6:305–306. doi: 10.1038/sj.gt.3300885. [DOI] [PubMed] [Google Scholar]
- 11.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayan A, Martin DR, Sadow JL, Kissane J, Miller SB. Hepatocyte growth factor inhibits apoptosis after ischemic renal injury in rats. Am J Kidney Dis. 2001;38:274–278. doi: 10.1053/ajkd.2001.26087. [DOI] [PubMed] [Google Scholar]
- 14.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 15.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 16.Chi CH, Wang YS, Lai YS, Chi KH. Anti-tumor effect of in vivo IL-2 and GM-CSF electrogene therapy in murine hepatoma model. Anticancer Res. 2003;23:315–321. [PubMed] [Google Scholar]
- 17.Chi KH, Wang HE, Wang YS, Chou SL, Yu HM, Tseng YH, Hwang IM, Lui WY. Antisense thymidylate synthase electrogene transfer to increase uptake of radiolabeled iododeoxyuridine in a murine model. J Nucl Med. 2004;45:478–484. [PubMed] [Google Scholar]
- 18.Yamashita YI, Shimada M, Hasegawa H, Minagawa R, Rikimaru T, Hamatsu T, Tanaka S, Shirabe K, Miyazaki JI, Sugimachi K. Electroporation-mediated interleukin-12 gene therapy for hepatocellular carcinoma in the mice model. Cancer Res. 2001;61:1005–1012. [PubMed] [Google Scholar]
- 19.Suzuki T, Shin BC, Fujikura K, Matsuzaki T, Takata K. Direct gene transfer into rat liver cells by in vivo electroporation. FEBS Lett. 1998;425:436–440. doi: 10.1016/s0014-5793(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 20.Xue F, Takahara T, Yata Y, Minemura M, Morioka CY, Takahara S, Yamato E, Dono K, Watanabe A. Attenuated acute liver injury in mice by naked hepatocyte growth factor gene transfer into skeletal muscle with electroporation. Gut. 2002;50:558–562. doi: 10.1136/gut.50.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao C, Jokerst R, Gondipalli P, Cai SR, Kennedy S, Ponder KP. Intramuscular injection of an adenoviral vector expressing hepatocyte growth factor facilitates hepatic transduction with a retroviral vector in mice. Hum Gene Ther. 1999;10:911–922. doi: 10.1089/10430349950018319. [DOI] [PubMed] [Google Scholar]
- 22.Matsuno Y, Iwata H, Umeda Y, Takagi H, Mori Y, Kosugi A, Matsumoto K, Nakamura T, Hirose H. Hepatocyte growth factor gene transfer into the liver via the portal vein using electroporation attenuates rat liver cirrhosis. Gene Ther. 2003;10:1559–1566. doi: 10.1038/sj.gt.3302052. [DOI] [PubMed] [Google Scholar]
- 23.Lewin K, Millis RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21–26. [PubMed] [Google Scholar]
- 24.Reed GB, Cox AJ. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol. 1966;48:597–611. [PMC free article] [PubMed] [Google Scholar]
- 25.Geraci JP, Mariano MS, Jackson KL. Hepatic radiation injury in the rat. Radiat Res. 1991;125:65–72. [PubMed] [Google Scholar]
- 26.Hebard DW, Jackson KL, Christensen GM. The chronological development of late radiation injury in the liver of the rat. Radiat Res. 1980;81:441–454. [PubMed] [Google Scholar]
- 27.Murase T, Anscher MS, Petros WP, Peters WP, Jirtle RL. Changes in plasma transforming growth factor beta in response to high-dose chemotherapy for stage II breast cancer: possible implications for the prevention of hepatic veno-occlusive disease and pulmonary drug toxicity. Bone Marrow Transplant. 1995;15:173–178. [PubMed] [Google Scholar]
- 28.Fan S, Ma YX, Wang JA, Yuan RQ, Meng Q, Cao Y, Laterra JJ, Goldberg ID, Rosen EM. The cytokine hepatocyte growth factor/scatter factor inhibits apoptosis and enhances DNA repair by a common mechanism involving signaling through phosphatidyl inositol 3' kinase. Oncogene. 2000;19:2212–2223. doi: 10.1038/sj.onc.1203566. [DOI] [PubMed] [Google Scholar]
- 29.Fan S, Wang JA, Yuan RQ, Rockwell S, Andres J, Zlatapolskiy A, Goldberg ID, Rosen EM. Scatter factor protects epithelial and carcinoma cells against apoptosis induced by DNA-damaging agents. Oncogene. 1998;17:131–141. doi: 10.1038/sj.onc.1201943. [DOI] [PubMed] [Google Scholar]
- 30.Maj JG, Paris F, Haimovitz-Friedman A, Venkatraman E, Kolesnick R, Fuks Z. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003;63:4338–4341. [PubMed] [Google Scholar]
- 31.Bickel M, Baringhaus KH, Gerl M, Günzler V, Kanta J, Schmidts L, Stapf M, Tschank G, Weidmann K, Werner U. Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology. 1998;28:404–411. doi: 10.1002/hep.510280217. [DOI] [PubMed] [Google Scholar]
- 32.Bedossa P, Paradis V. Transforming growth factor-beta (TGF-beta): a key-role in liver fibrogenesis. J Hepatol. 1995;22:37–42. [PubMed] [Google Scholar]
- 33.Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24:636–642. doi: 10.1053/jhep.1996.v24.pm0008781336. [DOI] [PubMed] [Google Scholar]
- 34.Geraci JP, Mariano MS. Radiation hepatology of the rat: parenchymal and nonparenchymal cell injury. Radiat Res. 1993;136:205–213. [PubMed] [Google Scholar]
- 35.Date M, Matsuzaki K, Matsushita M, Tahashi Y, Furukawa F, Inoue K. Modulation of transforming growth factor beta function in hepatocytes and hepatic stellate cells in rat liver injury. Gut. 2000;46:719–724. doi: 10.1136/gut.46.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]


