Abstract
AIM: The utility of serum alpha-fetoprotein (α-FP) for the detection of hepatocellular carcinoma (HCC) is questionable. High serum levels of chromogranin-A (CgA) have recently been reported in HCC. Impaired hepatic, renal, and heart functions influence circulating CgA. The aim of this study was to assess sensitivity and specificity of serum CgA as a marker of HCC in patients with liver cirrhosis (LC).
METHODS: Serum CgA levels were measured by RIA in 339 patients of which 54 HCC, 132 LC, 45 chronic hepatitis (CH), 27 chronic heart failure (CHF), 36 chronic renal failure (CRF), 45 chronic inflammatory bowel disease (IBD) as disease controls and in 75 healthy controls. Patients with liver disease or IBD and concomitant renal and/or heart failure were excluded. Pearson correlation, non-parametric combination test and confidence interval analysis were used for statistical analysis.
RESULTS: Serum CgA above normal values (100 ng/mL) were found in 83% of HCC patients, in 48% of LC patients, in 20% of CH patients, in 33% of IBD patients, in 92% of CRF patients, in 100% of CHF patients, and in none of the healthy controls. The mean CgA values in HCC (769±1 046), in LC (249±369), in CH (87±94), in CRF (1390±1401), in CHF (577±539), in IBD (146±287) were significantly higher than those in healthy controls (48±18). HCC patients had higher CgA values (P<0.01) than LC, CH, and IBD patients but did not differ from those with CRF or CHF. The 95% CI for the mean (250-1289 ng/mL) in HCC patients was selected as a CgA range and the lower value of such range was assumed as cut-off. Sensitivity and specificity of CgA, calculated in relation to the cut-off in patients with cirrhosis and HCC, were respectively 61% (CI 48-73%) and 82% (CI 75-88%). Serum α-FP values were >200 ng/mL in 21% of the HCC patients and in none of the LC patients. No significant correlation was found between α-FP and CgA in patients with HCC and in patients with cirrhosis.
CONCLUSION: When HCC is suspected and α-FP is normal or <200 ng/mL, CgA serum values represent a complementary diagnostic tool, unless kidney or heart failure is present.
Keywords: Chromogranin-A, Hepatocellular carcinoma, Liver cirrhosis, Chronic hepatitis, Diagnosis
INTRODUCTION
Chromogranin-A (CgA) is a 50-ku acid glycoprotein originally described in catecholamine storage vesicles of the adrenal medulla[1]. It has a wide distribution in secretory vesicles of the endocrine, neuroendocrine and nervous system, where it is co-stored and co-secreted with hormones and neurotransmitters[2] . It is present in low concentration in the serum of healthy individuals, while high serum levels represent a sensible marker of carcinoid-like tumors and neuroendocrine tumors such as neuroblastoma, pheochromocytoma, and small cell lung carcinoma[3,4] .
Recently, cellular clones with morphological and functional neuroendocrine features have been demonstrated in non-endocrine tumors, and elevated levels of serum CgA have been described in patients with carcinoma of the prostate, breast, ovary, pancreas, and colon[5] .
Cirrhosis of the liver is the most important risk factor for hepatocellular carcinoma (HCC), which represents the leading cause of death in these patients[6]. The most useful serum marker of the tumor, alpha-fetoprotein (α-FP), has a highly variable sensitivity and specificity in the reported studies[6,7]. The diagnostic value of other markers of HCC proposed so far, serum des-γ-carboxyprothrombin[8], p53 autoantibodies, TGF-β-1, α-L-fucosidase, glycilproline dipeptidyl aminopeptidase isoenzyme, nociceptin, is questionable.
Clusters of cells containing CgA have been demonstrated within HCC tissue[9-11]. One recent study[12], reporting high serum CgA values in approximately half patients with HCC, suggests that CgA might represent a useful marker of HCC. However, other factors, in particular renal and cardiac function, may affect circulating CgA, and elevated levels of serum CgA have been reported both in kidney failure[13] and in heart failure[14].
The aim of this study was to assess the sensitivity and specificity of serum CgA as a marker of HCC in patients with cirrhosis of the liver.
MATERIALS AND METHODS
Patients and healthy subjects
CgA serum values in a population of 339 consecutive elective patients, 54 with HCC, 132 with cirrhosis of the liver (LC), 45 with chronic hepatitis (CH), 27 with chronic heart failure (CHF), 36 with chronic renal failure (CRF), and 45 with newly diagnosed chronic inflammatory bowel disease (IBD) as disease controls, were studied. Serum CgA was also measured in 75 blood donors constituting the healthy control group. Patients with liver disease or IBD and concomitant renal and/or heart failure were not included in the study. The diagnosis of HCC was based on findings of imaging techniques and/or histological confirmation. The diagnosis of LC was clinical and/or histological. The diagnosis of CH was also histologically confirmed. The demographic and clinical characteristics of the populations studied are illustrated in Table 1.
Table 1.
Clinical-demographic data and serum CGA values.
| HCC | LC | CH | CRF | CHF | IBD | Controls | |
| Number of patients | 54 | 132 | 45 | 36 | 27 | 45 | 75 |
| Male/female | 39/15 | 93/39 | 30/15 | 18/18 | 12/15 | 30/15 | 51/24 |
| Age (yr) | 69±11 | 59±12 | 52±13 | 71±17 | 72±15 | 45±6 | 48±15 |
| CgA (ng/mL) | 769±1 046 | 249±369 | 87±94 1 | 390±1 401 | 577±539 | 146±287 | 48±18 |
| α-FP (ng/mL) | 79±160 | 10±13 | |||||
| Child–Pugh class | |||||||
| A | 12 | 36 | |||||
| B | 15 | 57 | |||||
| C | 27 | 39 |
Abbreviations: HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; IBD, inflammatory bowel disease; CRF, chronic renal failure; CHF, chronic heart failure.
Assays
Serum CgA was detected in deep frozen sera (-20 °C) using a commercial solid-phase two-site immunoradiometric assay, CGA-RIA CT, CIS Bio International, Schering S.A., GIF-Sur-Yvette, Cedex, France. Briefly, two monoclonal antibodies were prepared against sterically remote sites on the CgA molecule. The first one was coated in the solid phase, the second one was radio labeled with iodine 125. CgA molecules or fragments present in the samples to be tested were sandwiched between the two antibodies. After the formation of the coated antibody/antigen/iodinized antibody sandwich, the unbound tracer was removed by washing. The remaining radioactivity was proportional to the concentration of CgA in the sample. Same subject samples were evaluated in duplicate in the same assay. The normal range for serum levels of CgA reported in normal population is 20-100 ng/mL.
In patients with LC and with HCC α-FP serum values were determined with routine radioimmunoassay.
Statistical analysis
Interaction between variables was studied using Pearson correlation. Differences in CgA serum values among the groups were evaluated by non-parametric combination (NPC) test[15]. P<0.05 was considered as statistically significant. In the HCC group, a range of CgA values was constructed. In relation to the lower limit of such range, sensitivity and specificity of attribution of subjects with HCC and cirrhosis were determined. For sensitivity and specificity, CI were also built.
Softwares used were Methodologica S.R.L. (2001) for non-parametric analysis NPC test, confidence interval analysis (CIA) Windows version 2.0 (2000) for sensitivity and specificity evaluation, and SPSS, Windows 11.0 (2001) for box-plot construction.
RESULTS
Circulating levels of CgA above normal values, 100 ng/mL, were found in 83% of HCC patients, in 48% of LC patients, in 20% of CH patients, in 33% of IBD patients, in 92% of CRF patients, in 100% of CHF patients, and in none of the healthy subjects.
In all groups of patients, CgA values were not correlated with anagraphical age (Pearson test). Within each group no significant differences in CgA values were found between males and females (NPC test).
All groups of patients had serum CgA higher than controls (Table 1 and Figure 1). Statistical analysis performed by NPC test showed a highly significant difference (P = 0.000) of CgA values among the groups examined altogether. In order to ascertain which groups were imputable for the significance, all possible comparisons between paired groups were performed. The P values of the analysis are shown in Table 2. In particular, patients with HCC had CgA values higher than patients with cirrhosis or with CH but not different from those with renal failure or heart failure.
Figure 1.
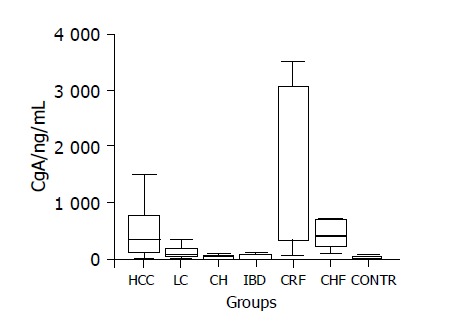
Box-plot diagram showing the distribution of CgA values in the groups studied. Abbreviations: HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; IBD, inflammatory bowel disease; CRF, chronic renal failure; CHF, chronic heart failure; CONTR, controls.
Table 2.
P values relative to comparison of serum CgA levels between paired groups (NPC test).
| Groups | HCC | LC | CH | IBD | CRF | CHF |
| L C | 0.004 | |||||
| CH | 0.000 | 0.167 | ||||
| IBD | 0.004 | 0.171 | 0.817 | |||
| CRF | 0.100 | 0.768 | 0.000 | 0 | ||
| CHF | 0.333 | 0.025 | 0.000 | 0.004 | 0.057 | |
| Controls | 0.000 | 0.000 | 0.022 | 0.000 | 0.000 | 0 |
Abbreviations: HCC, hepatocellular carcinoma; LC, liver cirrhosis; CH, chronic hepatitis; IBD, inflammatory bowel disease; CRF, chronic renal failure; CHF, chronic heart failure.
In order to define to which extent CgA values may be considered a marker of the presence of HCC in cirrhotic patients, the 95%CI for the mean (250-1289 ng/mL) was selected as CgA range in patients with HCC, and the lower value of such range was assumed as cut-off. Sensitivity and specificity of CgA, calculated in relation to the cut-off in patients with cirrhosis and HCC, were respectively 61% (CI 48-73%) and 82% (CI 75-88%)[16].
Serum α-FP levels >200 ng/mL were found in 21% of the patients with HCC and in none of the patients with LC. No significant correlation was found between α-FP values and CgA values in patients with HCC (P = 0.86) and in patients with cirrhosis (P = 0.50).
DISCUSSION
CgA is a sensitive marker of neuroendocrine tumors, valid for the diagnosis and follow up[17-19]. High serum levels of CgA have also been reported in non-neuroendocrine neoplasias, such as colon, lung, breast and prostate cancer, probably in relation to a neuroendocrine differentiation[20-23]. In a recent study, Leone et al[12] reported elevated CgA serum values in 43% of patients with cirrhosis and superimposed HCC suggesting that raising CgA levels, likely due to a neuroendocrine component of the tumor, might be a useful prognostic marker for HCC in cirrhotic patients. However, high serum CgA values are also found in patients with hepatic failure[24], CRF[25] and CHF[26], possibly because of inadequate hepatic metabolization, reduced renal elimination and neuroendocrine activation.
In this study, we found that patients with HCC had CgA values higher than patients with other liver disease, cirrhosis or CH, but not different from those with renal failure or heart failure. This finding suggests that determination of CgA serum values is useful in monitoring patients with cirrhosis of the liver for early detection of HCC, unless kidney or heart failure is present.
In order to determine the sensitivity and the specificity of the test for HCC detection, we constructed a range of CgA values in HCC and assumed as cut-off in the lower value of the range (250 ng/mL). This cut-off appears to have a good sensitivity and specificity, respectively 61% and 82%, for detection of HCC in patients with LC.
α-FP is the most commonly used circulating marker for HCC. In our series, as in others, a small percentage (21%) of patients with HCC had α-FP serum values >200 ng/mL, a cut-off considered suggestive for the presence of the tumor. Values of α-FP were not correlated with CgA values. Therefore, when α-FP is normal or <200 ng/mL and in the presence of suspicious clinical, laboratory and/or imaging signs of HCC, the evaluation of CgA levels becomes of particular importance in the follow-up of chronic liver disease patients.
Since patients with chronic liver disease and HCC with concomitant heart or kidney failure were not included in this study, in order to avoid the interference of these pathologies on the diagnostic value of CgA in HCC, further studies are necessary for the evaluation of the specificity of the marker in the entire population of chronic liver disease.
A follow-up of CgA serum values after treatment of HCC is recommended in order to define the utility of the marker for the detection of recurrent tumor.
ACKNOWLEDGMENTS
We are very grateful to Professor Fortunato Munaò of the Department of Statistics of our university who died recently. His relevant contribution was of extreme importance in the statistical analysis of this paper.
We would also like to thank Dr. Maria Concettina Tripoli for her significant contribution in the revision of the language.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967;215:58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- 2.Mouland AJ, Bevan S, White JH, Hendy GN. Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression. J Biol Chem. 1994;269:6918–6926. [PubMed] [Google Scholar]
- 3.Stridsberg M, Oberg K, Li Q, Engström U, Lundqvist G. Measurements of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. J Endocrinol. 1995;144:49–59. doi: 10.1677/joe.0.1440049. [DOI] [PubMed] [Google Scholar]
- 4.Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622–2628. doi: 10.1210/jcem.82.8.4145. [DOI] [PubMed] [Google Scholar]
- 5.Wu JT, Erickson AJ, Tsao KC, Wu TL, Sun CF. Elevated serum chromogranin A is detectable in patients with carcinomas at advanced disease stages. Ann Clin Lab Sci. 2000;30:175–178. [PubMed] [Google Scholar]
- 6.Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol. 2001;7:208–215. doi: 10.3748/wjg.v7.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebo KA, Chander G, Jenckes MW, Ghanem KG, Herlong HF, Torbenson MS, El-Kamary SS, Bass EB. Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C: a systematic review. Hepatology. 2002;36:S84–S92. doi: 10.1053/jhep.2002.36817. [DOI] [PubMed] [Google Scholar]
- 8.Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology. 1993;18:990–997. doi: 10.1002/hep.1840180434. [DOI] [PubMed] [Google Scholar]
- 9.Tajima Y, Nakajima T, Sugano I, Nagao K, Kondo Y, Saito J. Hepatocellular carcinoma containing endocrine cells. An autopsy report of triplecancer involving the liver, kidney and thyroid. Acta Pathol Jpn. 1992;42:904–910. doi: 10.1111/j.1440-1827.1992.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 10.Roskams T, Willems M, Campos RV, Drucker DJ, Yap SH, Desmet VJ. Parathyroid hormone-related peptide expression in primary and metastatic liver tumours. Histopathology. 1993;23:519–525. doi: 10.1111/j.1365-2559.1993.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima K, Sakurai A, Katai M, Yajima H, Mori J, Katakura M, Tsuchiya S, Hashizume K. Chromogranin A expression in hepatocellular carcinoma in a patient with germline MEN1 gene mutation. Intern Med. 2000;39:20–24. doi: 10.2169/internalmedicine.39.20. [DOI] [PubMed] [Google Scholar]
- 12.Leone N, Pellicano R, Brunello F, Rizzetto M, Ponzetto A. Elevated serum chromogranin A in patients with hepatocellular carcinoma. Clin Exp Med. 2002;2:119–123. doi: 10.1007/s102380200016. [DOI] [PubMed] [Google Scholar]
- 13.Tramonti G, Ferdeghini M, Annichiarico C, Norpoth M, Donadio C, Bianchi R, Bianchi C. Relationship between renal function and blood level of chromogranin A. Ren Fail. 2001;23:449–457. doi: 10.1081/jdi-100104728. [DOI] [PubMed] [Google Scholar]
- 14.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 15.Pesarin F. Multivariate permutation tests: with application in biostatistic. Chichester, New York, Weinheim, Brisbane, Singapore, Toronto: John Wiley & sons; 2001. pp. 1–408. [Google Scholar]
- 16.Altman DG. Diagnostic tests. In: Altman DG, Machin D, Bryant TN, Gardner MJ, eds , et al., editors. Statistics with confidence intervals and statistical guidelines. London UK: BMA House; 2000. pp. 109–123. [Google Scholar]
- 17.O'Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986;314:1145–1151. doi: 10.1056/NEJM198605013141803. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari L, Seregni E, Bajetta E, Martinetti A, Bombardieri E. The biological characteristics of chromogranin A and its role as a circulating marker in neuroendocrine tumours. Anticancer Res. 1999;19:3415–3427. [PubMed] [Google Scholar]
- 19.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 20.Syversen U, Halvorsen T, Mårvik R, Waldum HL. Neuroendocrine differentiation in colorectal carcinomas. Eur J Gastroenterol Hepatol. 1995;7:667–674. [PubMed] [Google Scholar]
- 21.Sobol RE, O'Connor DT, Addison J, Suchocki K, Royston I, Deftos LJ. Elevated serum chromogranin A concentrations in small-cell lung carcinoma. Ann Intern Med. 1986;105:698–700. doi: 10.7326/0003-4819-105-5-698. [DOI] [PubMed] [Google Scholar]
- 22.Giovanella L, Marelli M, Ceriani L, Giardina G, Garancini S, Colombo L. Evaluation of chromogranin A expression in serum and tissues of breast cancer patients. Int J Biol Markers. 2001;16:268–272. doi: 10.1177/172460080101600408. [DOI] [PubMed] [Google Scholar]
- 23.Deftos LJ, Abrahamsson PA. Granins and prostate cancer. Urology. 1998;51:141–145. doi: 10.1016/s0090-4295(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor DT, Pandlan MR, Carlton E, Cervenka JH, Hslao RJ. Rapid radioimmunoassay of circulating chromogranin A: in vitro stability, exploration of the neuroendocrine character of neoplasia, and assessment of the effects of organ failure. Clin Chem. 1989;35:1631–1637. [PubMed] [Google Scholar]
- 25.Hsiao RJ, Mezger MS, O'Connor DT. Chromogranin A in uremia: progressive retention of immunoreactive fragments. Kidney Int. 1990;37:955–964. doi: 10.1038/ki.1990.71. [DOI] [PubMed] [Google Scholar]
- 26.Corti A, Ferrari R, Ceconi C. Chromogranin A and tumor necrosis factor-alpha (TNF) in chronic heart failure. Adv Exp Med Biol. 2000;482:351–359. doi: 10.1007/0-306-46837-9_28. [DOI] [PubMed] [Google Scholar]


