Abstract
AIM: To investigate the effects of psychological stress on small intestinal motility and bacteria and mucosa in mice, and to explore the relationship between small intestinal dysfunction and small intestinal motility and bacteria and mucosa under psychological stress.
METHODS: Sixty mice were randomly divided into psychological stress group and control group. Each group were subdivided into small intestinal motility group (n = 10), bacteria group (n = 10), and D-xylose administered to stomach group (n = 10). An animal model with psychological stress was established housing the mice with a hungry cat in separate layers of a two-layer cage. A semi-solid colored marker (carbon-ink) was used for monitoring small intestinal transit. The proximal small intestine was harvested under sterile condition and processed for quantitation for aerobes (Escherichia coli) and anaerobes (Lactobacilli). The quantitation of bacteria was expressed as log10(colony forming units/g). D-xylose levels in plasma were measured for estimating the damage of small intestinal mucosa.
RESULTS: Small intestinal transit was inhibited (39.80±9.50% vs 58.79±11.47%, P<0.01) in mice after psychological stress, compared with the controls. Psychological stress resulted in quantitative alterations in the aerobes (E. coli). There was an increase in the number of E. coli in the proximal small intestinal flora (1.78±0.30 log10(CFU/g) vs 1.37±0.21 log10(CFU/g), P<0.01), and there was decrease in relative proportion of Lactobacilli and E. coli of stressed mice (0.53±0.63 vs 1.14±1.07, P<0.05), while there was no significant difference in the anaerobes (Lactobacilli) between the two groups (2.31±0.70 log10(CFU/g) vs 2.44±0.37 log10(CFU/g), P>0.05). D-xylose concentrations in plasma in psychological stress mice were significantly higher than those in the control group (2.90±0.89 mmol/L vs 0.97±0.33 mmol/L, P<0.01).
CONCLUSION: Small intestinal dysfunction under psychological stress may be related to the small intestinal motility disorder and dysbacteriosis and the damage of mucosa probably caused by psychological stress.
Keywords: Psychological stress, Small intestinal motility, Small intestinal bacteria, Small intestinal mucosa
INTRODUCTION
In 1972 Levi et al, established that psychosocial factors such as stressors could result in physiological disease. This has attracted much clinical interest. The role that psychosocial factors play in disease is gradually being recognized. It is believed that psychological stress could alter the function of small intestine and large intestine in psychological research. Alternative gastrointestinal function under stress may be associated with functional dyspepsia and irritable bowel syndrome (IBS). And IBS has been described as classical psychosomatic disorders by psychosomaticist.
The effect of psychological and physical stress on intestinal motility is different[1,2]. Previous studies of psychological stress[3,4] (cold stress, restraint stress, footshock stress) were all focused on physical stress.
Psychological stress modulates gut function. Sustained and acute life-threatening stressors play an important role in the onset and modulation of GI symptoms as well as in the development of affective disorders and posttraumatic stress disorder. We established an animal model with psychological stress housing mice with a hungry cat in separate layers of a two-layer cage. Our aim was to determine the effect of psychological stress on small intestinal motility and bacteria and mucosa, and discuss the mechanism that psychological stress may cause the dysfunction in small bowel.
MATERIALS AND METHODS
Materials
Sixty healthy male mice (provided by Qinglongshan Experimental Animal Center) weighing 20-30 g were used in this study. Mice were housed individually in cages at constant room temperature in a 12-h light/dark cycle and had free access to laboratory feed and water. Bacteria evaluation kit (API 20) was purchased from French Biomerieux. D-xylose kit was purchased from NanJing Jiancheng Bioengineering Institute (NJBI).
Methods
Establishment of animal model Sixty mice were randomly divided into psychological stress group and control group. Each group were subdivided into small intestinal motility group (n = 10) and bacteria group (n = 10) and D-xylose administered into stomach group (n = 10). Mice in psychological stress group were housed in the bottom of the two-layer cage, with a hungry cat being housed in the proximal layer of the cage for 10 min each day for 15 d, but mice and the cat had no physical contact. Procedure of the control group mice was as same as psychological stress group except for no contact with the cat.
Measurement of small intestinal transit The carbon-ink transit test was modified as described. Mice were deprived of food for 24 h and water for 12 h prior to experiment, and 0.3 mL carbon-ink (10% gum acacia, 5% activated charcoal) was administered into stomach by orogastric gavage. Twenty-five minutes later, the mice were killed, abdomen was opened and small intestine was dissected. The total length of the small intestine (pylorus-cecum) and the distance traveled by carbon-ink were measured. Results were expressed as ratio (%) of the distance traveled by carbon-ink to the total length of the small intestine.
Measurement of small intestinal bacteria Mice were deprived of food for 24 h and water for 12 h prior to experiment. The mice were killed, abdomen was opened and the proximal small intestine was harvested under sterile condition. The 2-cm-long small intestine, which was dissected at about a 10-cm point from pylorus was rinsed with sterile saline thrice, and then the leftover was sucked by sterile filter paper. After weighing, the small intestine with 2 mL sterile saline was placed in a sterile glass homogenizer and homogenized. Homogenate was diluted with sterile saline at different ratios and 100 μL dilution was plated on SS agar (Escherichia coli) and MRS agar (Lactobacilli). Quantity of E. coli was determined after 24 h of incubation at 37 °C. Quantity of Lactobacilli was determined after 48 h of incubation at 37 °C. Colony forming units (CFU) of bacteria were quantified by counting CFU from agar. The quantity of bacteria was expressed as log10(CFU/g). Quality of aseptic manipulation was evaluated by swab of abdominal cavity inoculating on sheep-blood agar.
Measurement of D-xylose concentrations in plasma Mice were deprived of food for 24 h and water for 12 h prior to experiment, and 0.4 mL 5% D-xylose solution was administered into stomach by orogastric gavage. One hour later, blood samples were collected into chilled tubes containing 100 U heparin immediately after the mice were killed. The blood was centrifuged at 3000 r/min at 4 °C for 10 min. The plasma was stored at -70 °C until assayed. Levels of D-xylose in plasma were measured with D-xylose kit.
Statistical analysis
Throughout this report, data were expressed as mean±SD. Experimental results were analyzed by t test. P<0.05 was considered statistically significant.
RESULTS
Small intestinal transit
Figure 1 presents data for the overall mean ratio of small intestinal transit (percentage of the distance traveled by intragastric carbon-ink to the total length of the small intestine after 25 min). The overall mean ratio of small intestinal transit under psychological stress was lower than that of the control (39.80±9.50% compared with 58.79±11.47%, P<0.01), indicating that psychological stress could inhibit small intestinal transit.
Figure 1.
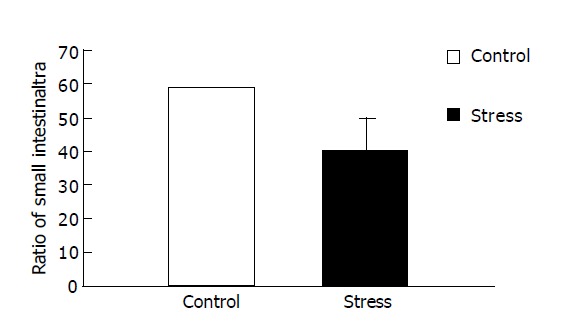
Effect of psychological stress on small intestinal transit.
Small intestinal bacteria
Psychological stress resulted in quantitative alterations in the aerobes (E. coli). The number of E. coli in the proximal small intestinal flora were more than those of the control (1.78±0.30 log10(CFU/g) compared with 1.37±0.21 log10(CFU/g), P<0.01), and the relative proportion of Lactobacilli and E. coli of stressed mice was lower than that of the control (0.53±0.63 compared with 1.14±1.07, P<0.01), while there was no statistically significant difference in the anaerobes (Lactobacilli) between the two groups (2.31±0.70 log10(CFU/g) compared with 2.44±0.37 log10(CFU/g). The data indicated that psychological stress could result in E. coli overgrowth and dysbacteriosis (Table 1).
Table 1.
Change of small intestinal bacteria (log10(CFU/g), mean±SD, n = 10).
| E. coli | Lactobacilli | L/E | |
| Control | 1.37±0.21 | 2.44±0.37 | 1.14±1.07 |
| Stress | 1.78±0.30b | 2.31±0.70 | 0.53±0.63a |
P<0.05,
P<0.01 vs the control.
D-xylose concentrations in plasma
D-xylose concentrations in plasma in psychologically stressed mice were significantly higher than those in the control group (2.90±0.89 mmol/L compared with 0.97±0.33 mmol/L, P<0.01), indicating that small intestinal mucosa was damaged (Figure 2).
Figure 2.
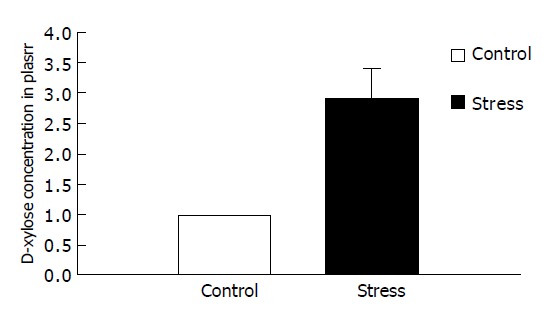
Changes of D-xylose in plasma.
DISCUSSION
The association between psychological stress and small intestinal motility has been postulated for about 20 years. Some studies in experimental animals indicated contradictory results, which may be due to different stressors in part. Varied stressors can influence small intestinal motility via different mechanisms. Muelas et al[5], demonstrated that restraint stress increased small intestinal motility both during fasting and after food. But restraint stress completely abolished the migrating motor complex (MMC)-interdigestive myoelectric complex periodicity characteristic of the normal fasting pattern. Ditto et al[6], reported that a prolonged active coping stressor with minimal motor requirements enhanced small intestinal transit. However, Tsukada et al[7-9], demonstrated that the small intestinal transit was significantly inhibited by restraint stress but not by footshock stress. And footshock stimulus may cancel the inhibition of small intestinal motility by restraint stress. In the present study, we demonstrated that the ratio of small intestinal transit was significantly decreased by psychological stress.
Host and intestinal normal flora are in a state of balance. Varied microorganisms of intestinal normal flora interact to keep themselves in a state of balance too. Changes of host and external environment could lead to disequilibrium. Itoh and Freter[10] demonstrated that the Lactobacilli in gnotobiotic mice suppressed E. coli multiplication in the stomach and the small intestine, but had no demonstrable effect on E. coli multiplication in the large intestine. Stress can cause significant change of intestinal flora. Lizko[11,12] revealed the factors that are neuroemotional tension, hypokinesia, increased physical load, isolation under conditions of altered gaseous environment and microclimate participated in development of dysbacteriosis under extreme conditions (space flights of various duration). Intestinal microflora in man responded by decreased counts of bifidobacteria and Lactobacilli participating in maintenance of the intact ecological barrier and colonization resistance. Furthermore, the neuroemotional stress played the main role in development of dysbacteriosis in man under extreme conditions, which was supported by the following study. During the preparation phase of the flight and the period immediately before the take-off and after the flight, there was a distinct decrease in the numbers of Bifidobacterium and Lactobacilli as well as a substantial increase in the numbers of E. coli. This seems to be due to nervous-emotional stress effects. Logan et al[13], reported that patients with chronic fatigue syndrome (CFS) had marked alterations in microbial flora, including lowered levels of bifidobacteria and small intestinal bacterial overgrowth (SIBO). Bailey and Coe[14] reported that maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Fecal bacteria decrease significantly, especially Lactobacilli. The drop in the microflora was correlated with the display of stress-indicative behaviors, but not with cortisol secretion. In addition, infants who displayed numerous stress-indicative behaviors were more susceptible to opportunistic bacterial infection. Gritsenko et al[15], revealed that 6-h immobilization stress initiates the increase of the concentration of E. coli in the proximal sections of the digestive tract (the duodenum and the jejunum). Ringo et al[16], reported that the total culturable bacterial numbers or population level of the lactic acid bacteria associated with the digestive tract of Atlantic salmon (Salmo salar L.) had no significant alteration under excessive handling stress and starvation. In the present study, the results were similar to the above research. After psychological stress, the number of E. coli of the proximal small intestine in mice increased significantly compared to the control. The number of Lactobacilli was not statistically significant and different from the control. The ratio of Lactobacilli and E. coli was lower than that of the control. Glunder[17] demonstrated that stressful situations such as overcrowding in small cages coincident with increased noise and low light levels can enhance the colonization of the gut with E. coli. The present study was involved in stressful situations because all of the mice were placed in the cage when they were under stress. Lencner et al[18]. reported that the lactoflora of cosmonauts showed distinct changes due to the emotional stress before the take off. Compared to long-time flights, short-time flights caused even stronger alterations of lactoflora. The reason was that the disturbance that took place before the take-off as to the short time could not be balanced by the macroorganism. Additionally, after a certain adaptive period the factors of the space travel began to act. The reason may be the explanation that there was no difference in the number of Lactobacilli of small intestine between the stressed mice and the control in the present study.
Leveau et al[19], demonstrated that impairment in intestinal motility probably played a pathophysiological role in the development of bacterial overgrowth. A delay in intestinal transit time appeared as an early event in acute pancreatitis, preceding intestinal bacterial overgrowth. Gangarosa[20] demonstrated that intestinal motility served as a normal cleansing mechanism of the intestine, and drugs that decreased this motility might facilitate replication of pathogens and their attachment to or invasion of the intestinal tissue. Wang et al[21-23], demonstrated that delayed intestinal transit after hepatectomy might contribute to overgrowth of E. coli in small intestine. Administration of cisapride or CCK prevented overgrowth of E. coli by improving intestinal motility in rats.
Interdigestive small bowel motility has a regulatory function on the microflora of the upper small bowel. Nieuwenhuijs et al[24,25], clarified the role of the migrating motor complex (MMC) in the regulation of small intestinal microflora. The MMC is an important mechanism controlling bacterial growth in the upper small bowel. Its disruption with morphine promotes duodenal bacterial overgrowth. MMC can coordinate the movement of pylorus and gut and cholecyst. So the alteration of MMC necessarily has influence on the secretion of bile, of which bile can inhibit gram-negative bacilli. Grzesiuk et al[26], demonstrated that intestinal bacteria, particularly those adhering to intestinal epithelial cells, were exposed to electric fields and currents generated by the muscular activity of the small intestine. The myoelectrical activity of the duodenum, through action on cell membrane, can affect cell division of intestinal bacteria. The growth of E. coli stimulated by the electric current was significantly inhibited after a period of intensive growth.
Intestinal microflora can modulate myoelectric activity of small intestine. Husebye et al[27,28], demonstrated that after introduction of conventional intestinal microflora the interval between activity fronts of the migrating myoelectric complex in proximal jejunum of germ-free rats was reduced. Intestinal bacteria promoted or suppressed the initiation and aboral migration of the migrating myoelectric complex (MMC) depending on the species involved. Bacteria with primitive fermenting metabolism (anaerobes) emerge as important promoters of regular spike burst activity in small intestine. Lactobacillus promoted regular spike burst activity and reduced the MMC period and accelerated small intestinal transit. E. coli showed an inhibitory effect on MMC. Thorlacius et al[29], reported that Lactobacillus could enhance intestinal transit. Sjogren et al[30], demonstrated that bacterial adherence to the intestinal mucosa appeared to be important in eliciting the abnormal myoelectric responses. E. coli prolonged spike bursts. Tsafarov et al[31], demonstrated that E. coli inhibited the movements of the intestine, which was manifested by a reduction of the frequency and amplitude of the intestinal contractions. Cuoco et al[32], reported that bacterial overgrowth might contribute to the delay of intestinal transit. After eradication therapy, patients without bacterial overgrowth showed a significant reduction of oro-cecal transit time. Pimentel et al[33], demonstrated that the duration and frequency of phase III was reduced in subjects with irritable bowel syndrome (IBS) and SIBO. Subjects whose SIBO was still present had less phase III events than subjects with eradicated overgrowth. Eradication of bacterial overgrowth seems to result in some normalization of motility. The above can explain occurrence and development of some symptoms of IBS. Madrid et al[34], administrated patients with liver cirrhosis prokinetics (cisapride) or antibiotics. After 6-mo treatment, both cisapride and antibiotics significantly improved fasting cyclic activity, reduced the duration of oro-cecal transit time, and decreased SIBO. Cisapride administration was also followed by an increase in the amplitude of contractions.
Meddings and Swain[35] demonstrated that psychological stress might increase permeability of all regions of the gastrointestinal tract. This provides a potential mechanism for the observation of stress-induced disease recurrence in Crohn’s disease. Velin et al[36], reported that the barrier function of follicle-associated epithelium could be modulated. Chronic water avoidance stress further increased permeability of villus and follicle-associated epithelium than acute water avoidance stress. Saunders et al[37], reported that acute stress altered jejunal epithelial physiology. Both physical stress (2 h of cold-restraint stress) and psychological stress (1 h of water-avoidance stress) increased ionic and macromolecular permeability of intestine. Wilson and Baldwin[38] demonstrated that mental stress (environmentally induced stress) caused pathological changes in the rat intestinal mucosa, which compromise the epithelial-endothelial exchange barrier, where intestinal villi were edematous and epithelial cells were detaching from the basement membrane at villus tips. Santos et al[39], reported that chronic stress (water avoidance stress or sham stress (1 h/d) for 5 d) causes an epithelial barrier defect and epithelial mitochondrial damage. Soderholm et al[40], demonstrated that chronic psychological stress could be an initiating factor in intestinal inflammation by impairing mucosal defenses against luminal bacteria. Chronic stress (water avoidance stress or sham stress as a model of ongoing life stress) induced barrier dysfunction in the ileum and colon (increased macromolecular permeability and depletion of mucus) and ultrastructural changes in epithelial cells (enlarged mitochondria and presence of autophagosomes) associated with bacterial adhesion and penetration into enterocytes. Shi et al[41], reported that chronic restraint stress could cause damage on intestinal barrier function, increased intestinal permeability to D-xylose. The levels of D-xylose in plasma of stressed rats were higher than that of the control group. The result of our study showed that concentration of D-xylose in plasma of mice subjected to psychological stress was significantly higher than the control, which suggested that psychological stress caused damage of small intestinal mucosa and increased permeability to luminal substances.
Schiffrin et al[42], suggested that the developmental condition of the host’s intestinal barrier might be an important regulator of the bacterial microenvironment of the newborn small intestinal mucosa. Garcia-Lafuente et al[43], demonstrated that certain commensal bacteria could modify colonic wall permeability to luminal substances. E. coli significantly increased lumen to blood clearance. Colonization with Lactobacillus had the opposite effect and reduced permeability to mannitol. Isolauri et al[44], reported that Lactobacillus counteracted permeability disorder of the mucosal barrier. Logan et al[13], demonstrated that Lactobacillus might have a therapeutic role in the treatment of chronic fatigue syndrome (CFS). Lactobacillus are strong antioxidants, can enhance absorption of micronutrients by protecting the intestinal epithelial barrier, and have been used to treat SIBO. Bomba et al[45], suggested that the Lactobacilli-produced organic acids might present an efficient barrier inhibiting the adherence of digestive tract pathogens to the intestinal mucosa. Lievin-Le Moal et al[46], reported that Lactobacill isolated from the resident adult human gastrointestinal microflora, together with its antimicrobial activity, exerts a protective effect against the brush border lesions promoted by adhering E. coli in human intestinal cells. Ding et al[47], reported that increased E. coli caused the magnitude of gut mucosal injury. Zareie et al[48], reported that gram-negative luminal bacteria could cause significant alterations in epithelial ion transport and barrier functions. McNamara et al[49], clarified that E. coli disrupts intestinal barrier function, increased monolayer permeability and redistributed the tight junction-associated protein occludin. Perers et al[50], reported that E. coli damaged the brush border of the mucosal epithelium. Muza-Moons et al[51], reported that E. coli disrupted the structure and barrier function of host intestinal epithelial tight junctions (TJS). Michail et al[52], indicated that E. coli-induced neutrophil migration could occur without significant disruption of barrier function.
Gangarosa[53] demonstrated that intestinal motility served as a normal cleansing mechanism of the intestine, and drugs that decreased this motility might facilitate replication of pathogens and their attachment to or invasion of the intestinal tissue. Bacterial adherence to epithelia of intestinal mucosa plays an important role in the interaction of host and bacteria. Rocha et al[54], demonstrated that mucosa-associated E. coli played specific role on epithelial barrier dysfunction. Stress increased numbers of mucosa-associated E. coli in the cecum, which could increase epithelial permeability. E. coli of mice submitted to stress adhered to and altered the permeability of young adult mouse colon cells, whereas E. coli from the cecum of control mice were less adherent and had no effect on epithelial permeability.
Previous studies have shown that psychological stress can cause gastrointestinal dysfunction. For example, patients with gastrointestinal motility disorders, especially irritable bowel syndrome (IBS), symptoms of abdominal pain and bloating were precipitated after psychological stress (life events). Ford et al[55], demonstrated that anxiety resulting from psychological stress could enhance the colonic symptoms such as intestinal tympanites and abdominal distention. The mechanism may be explained by the fact that psychological stress induced decrease of small intestinal transit and overgrowth of bacteria in small intestine as well as damage of small intestinal mucosa. Intestinal gas could not be evacuated effectively and developed gas retention and which evoked a series of symptoms, especially pain and bloating[56]. Furthermore, the prolonged small intestinal transit makes it possible that the small intestinal content remains in the intestinal tract for a long time. Increasing bacteria in small intestine break the content down and produce a great deal of gas. The defect of intestinal barrier and the above factors can make symptoms become more serious. These aspects of small intestinal dysfunction may enhance each other.
The results of this study suggest that psychological stress can cause small intestinal dismotility and dysbacteriosis and a defect of intestinal barrier, which may be correlated to each other and interact. These results provide evidence to stimulate further research into the mechanisms linking mental stress to gastrointestinal dysfunction in humans.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985–990. doi: 10.1111/j.1572-0241.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 2.Morrow NS, Garrick T. Effects of intermittent tail shock or water avoidance on proximal colonic motor contractility in rats. Physiol Behav. 1997;62:233–239. doi: 10.1016/s0031-9384(97)00108-x. [DOI] [PubMed] [Google Scholar]
- 3.Dai Y, Liu JX, Li JX, Xu YF. Effect of pinaverium bromide on stress-induced colonic smooth muscle contractility disorder in rats. World J Gastroenterol. 2003;9:557–561. doi: 10.3748/wjg.v9.i3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagi S, Takaki A, Hori T, Sugimachi K. Enteric lipopolysaccharide raises plasma IL-6 levels in the hepatoportal vein during non-inflammatory stress in the rat. Fukuoka Igaku Zasshi. 2002;93:38–51. [PubMed] [Google Scholar]
- 5.Muelas MS, Ramírez P, Parrilla P, Ruiz JM, Pérez JM, Candel MF, Aguilar J, Carrasco L. Vagal system involvement in changes in small bowel motility during restraint stress: an experimental study in the dog. Br J Surg. 1993;80:479–483. doi: 10.1002/bjs.1800800424. [DOI] [PubMed] [Google Scholar]
- 6.Ditto B, Miller SB, Barr RG. A one-hour active coping stressor reduces small bowel transit time in healthy young adults. Psychosom Med. 1998;60:7–10. doi: 10.1097/00006842-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada F, Sawamura K, Kohno H, Ohkubo Y. Mechanism of inhibition of small intestinal motility by restraint stress differs from that with norepinephrine treatment in rats. Biol Pharm Bull. 2002;25:122–124. doi: 10.1248/bpb.25.122. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada F, Sugawara M, Kohno H, Ohkubo Y. Evaluation of the effects of restraint and footshock stress on small intestinal motility by an improved method using a radionuclide, 51Cr, in the rat. Biol Pharm Bull. 2001;24:488–490. doi: 10.1248/bpb.24.488. [DOI] [PubMed] [Google Scholar]
- 9.Tsukada F, Ohuchi Y, Terunuma T, Sugawara M, Kohno H, Ohkubo Y. Activation of mu-opioid pathway is associated with the canceling effect of footshock stimulus on the restraint stress-induced inhibition of small intestinal motility in rats. Biol Pharm Bull. 2001;24:1332–1334. doi: 10.1248/bpb.24.1332. [DOI] [PubMed] [Google Scholar]
- 10.Itoh K, Freter R. Control of Escherichia coli populations by a combination of indigenous clostridia and lactobacilli in gnotobiotic mice and continuous-flow cultures. Infect Immun. 1989;57:559–565. doi: 10.1128/iai.57.2.559-565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liz'ko NN. The dysbacteriosis of extreme states. Antibiot Med Biotekhnol. 1987;32:184–186. [PubMed] [Google Scholar]
- 12.Liźko NN, Silov VM, Syrych GD. Events in he development of dysbacteriosis of the intestines in man under extreme conditions. Nahrung. 1984;28:599–605. doi: 10.1002/food.19840280604. [DOI] [PubMed] [Google Scholar]
- 13.Logan AC, Venket Rao A, Irani D. Chronic fatigue syndrome: lactic acid bacteria may be of therapeutic value. Med Hypotheses. 2003;60:915–923. doi: 10.1016/s0306-9877(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 14.Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35:146–155. [PubMed] [Google Scholar]
- 15.Gritsenko VA, Brudastov IuA, Zhurlov OS, Chertkobv KL. The properties of Escherichia isolated from the bodies of mice in bacterial translocation after immobilization stress. Zh Mikrobiol Epidemiol Immunobiol. 2000;1:37–41. [PubMed] [Google Scholar]
- 16.Ringø E, Bendiksen HR, Wesmajervi MS, Olsen RE, Jansen PA, Mikkelsen H. Lactic acid bacteria associated with the digestive tract of Atlantic salmon (Salmo salar L.) J Appl Microbiol. 2000;89:317–322. doi: 10.1046/j.1365-2672.2000.01116.x. [DOI] [PubMed] [Google Scholar]
- 17.Glünder G. Influence of diet on the occurrence of some bacteria in the intestinal flora of wild and pet birds. Dtsch Tierarztl Wochenschr. 2002;109:266–270. [PubMed] [Google Scholar]
- 18.Lencner AA, Lencner CP, Mikelsaar ME, Tjuri ME, Toom MA, Väljaots ME, Silov VM, Liz'ko NN, Legenkov VI, Reznikov IM. The quantitative composition of the intestinal lactoflora before and after space flights of different lengths. Nahrung. 1984;28:607–613. doi: 10.1002/food.19840280608. [DOI] [PubMed] [Google Scholar]
- 19.Leveau P, Wang X, Soltesz V, Ihse I, Andersson R. Alterations in intestinal motility and microflora in experimental acute pancreatitis. Int J Pancreatol. 1996;20:119–125. doi: 10.1007/BF02825510. [DOI] [PubMed] [Google Scholar]
- 20.Gangarosa EJ. Recent developments in diarrheal diseases. Postgrad Med. 1977;62:113–117. doi: 10.1080/00325481.1977.11714583. [DOI] [PubMed] [Google Scholar]
- 21.Wang XD, Guo WD, Wang Q, Andersson R, Ekblad E, Soltesz V, Bengmark S. The association between enteric bacterial overgrowth and gastrointestinal motility after subtotal liver resection or portal vein obstruction in rats. Eur J Surg. 1994;160:153–160. [PubMed] [Google Scholar]
- 22.Wang XD, Soltesz V, Andersson R. Cisapride prevents enteric bacterial overgrowth and translocation by improvement of intestinal motility in rats with acute liver failure. Eur Surg Res. 1996;28:402–412. doi: 10.1159/000129484. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Soltesz V, Axelson J, Andersson R. Cholecystokinin increases small intestinal motility and reduces enteric bacterial overgrowth and translocation in rats with surgically induced acute liver failure. Digestion. 1996;57:67–72. doi: 10.1159/000201315. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwenhuijs VB, van Duijvenbode-Beumer H, Verheem A, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The effects of ABT-229 and octreotide on interdigestive small bowel motility, bacterial overgrowth and bacterial translocation in rats. Eur J Clin Invest. 1999;29:33–40. doi: 10.1046/j.1365-2362.1999.00364.x. [DOI] [PubMed] [Google Scholar]
- 25.Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998;228:188–193. doi: 10.1097/00000658-199808000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grzesiuk E, Laubitz D, Wójcik-Sikora A, Zabielski R, Pierzynowski SG. Influence of intestinal myoelectrical activity on the growth of Escherichia coli. Bioelectromagnetics. 2001;22:449–455. doi: 10.1002/bem.72. [DOI] [PubMed] [Google Scholar]
- 27.Husebye E, Hellström PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci. 1994;39:946–956. doi: 10.1007/BF02087542. [DOI] [PubMed] [Google Scholar]
- 28.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 29.Thorlacius H, Nobaek S, Wang XD, Andersson R, Molin G, Bengmark S, Jeppsson B. Lactobacilli attenuate bacteremia and endotoxemia associated with severe intra-abdominal infection. Surgery. 2003;134:467–473. doi: 10.1067/s0039-6060(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 30.Sjogren RW, Sherman PM, Boedeker EC. Altered intestinal motility precedes diarrhea during Escherichia coli enteric infection. Am J Physiol. 1989;257:G725–G731. doi: 10.1152/ajpgi.1989.257.5.G725. [DOI] [PubMed] [Google Scholar]
- 31.Tsafarov M. Changes in the motor activity of an isolated intestinal loop from the jejunum of dogs exposed to an endotoxin. Eksp Med Morfol. 1979;18:76–81. [PubMed] [Google Scholar]
- 32.Cuoco L, Montalto M, Jorizzo RA, Santarelli L, Arancio F, Cammarota G, Gasbarrini G. Eradication of small intestinal bacterial overgrowth and oro-cecal transit in diabetics. Hepatogastroenterology. 2002;49:1582–1586. [PubMed] [Google Scholar]
- 33.Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639–2643. doi: 10.1023/a:1021039032413. [DOI] [PubMed] [Google Scholar]
- 34.Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251–1255. doi: 10.1111/j.1572-0241.2001.03636.x. [DOI] [PubMed] [Google Scholar]
- 35.Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 36.Velin AK, Ericson AC, Braaf Y, Wallon C, Söderholm JD. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut. 2004;53:494–500. doi: 10.1136/gut.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002;47:208–215. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- 38.Wilson LM, Baldwin AL. Environmental stress causes mast cell degranulation, endothelial and epithelial changes, and edema in the rat intestinal mucosa. Microcirculation. 1999;6:189–198. [PubMed] [Google Scholar]
- 39.Santos J, Yang PC, Söderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Söderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- 41.Shi HL, Cheng YY, Li ST, Wang DL, Yu ZJ, Geng ZH, Chen WQ, Feng P. Effect of chronic restraint stress on intestinal barrier function of rats. Zhongguo Xingwei Yixue Kexue. 2003;12:251–253. [Google Scholar]
- 42.Schiffrin EJ, Carter EA, Walker WA, Frieberg E, Benjamin J, Israel EJ. Influence of prenatal corticosteroids on bacterial colonization in the newborn rat. J Pediatr Gastroenterol Nutr. 1993;17:271–275. [PubMed] [Google Scholar]
- 43.García-Lafuente A, Antolín M, Guarner F, Crespo E, Malagelada JR. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut. 2001;48:503–507. doi: 10.1136/gut.48.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105:1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- 45.Bomba A, Kastel R, Gancarclková S, Nemcová R, Herich R, Cizek M. The effect of lactobacilli inoculation on organic acid levels in the mucosal film and the small intestine contents in gnotobiotic pigs. Berl Munch Tierarztl Wochenschr. 1996;109:428–430. [PubMed] [Google Scholar]
- 46.Liévin-Le Moal V, Amsellem R, Servin AL, Coconnier MH. Lactobacillus acidophilus (strain LB) from the resident adult human gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut. 2002;50:803–811. doi: 10.1136/gut.50.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding J, Magnotti LJ, Huang Q, Xu DZ, Condon MR, Deitch EA. Hypoxia combined with Escherichia coli produces irreversible gut mucosal injury characterized by increased intestinal cytokine production and DNA degradation. Shock. 2001;16:189–195. doi: 10.1097/00024382-200116030-00004. [DOI] [PubMed] [Google Scholar]
- 48.Zareie M, Singh PK, Irvine EJ, Sherman PM, McKay DM, Perdue MH. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn's disease. Am J Pathol. 2001;158:1101–1109. doi: 10.1016/S0002-9440(10)64057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNamara BP, Koutsouris A, O'Connell CB, Nougayréde JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest. 2001;107:621–629. doi: 10.1172/JCI11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perers L, Andåker L, Edebo L, Stendahl O, Tagesson C. Association of some enterobacteria with the intestinal mucosa of mouse in relation to their partition in aqueous polymer two-phase systems. Acta Pathol Microbiol Scand B. 1977;85B:308–316. doi: 10.1111/j.1699-0463.1977.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 51.Muza-Moons MM, Koutsouris A, Hecht G. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect Immun. 2003;71:7069–7078. doi: 10.1128/IAI.71.12.7069-7078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michail SK, Halm DR, Abernathy F. Enteropathogenic Escherichia coli: stimulating neutrophil migration across a cultured intestinal epithelium without altering transepithelial conductance. J Pediatr Gastroenterol Nutr. 2003;36:253–260. doi: 10.1097/00005176-200302000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Gangarosa EJ. Recent developments in diarrheal diseases. Postgrad Med. 1977;62:113–117. doi: 10.1080/00325481.1977.11714583. [DOI] [PubMed] [Google Scholar]
- 54.Rocha F, Laughlin R, Musch MW, Hendrickson BA, Chang EB, Alverdy J. Surgical stress shifts the intestinal Escherichia coli population to that of a more adherent phenotype: role in barrier regulation. Surgery. 2001;130:65–73. doi: 10.1067/msy.2001.115360. [DOI] [PubMed] [Google Scholar]
- 55.Ford MJ, Camilleri M, Zinsmeister AR, Hanson RB. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology. 1995;109:1772–1780. doi: 10.1016/0016-5085(95)90743-2. [DOI] [PubMed] [Google Scholar]
- 56.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]


