Abstract
Few clinical studies have demonstrated an anti-proliferative activity of somatostatin (SST) analogs in carcinoids. We report the case of a woman with liver metastases of neuroendocrine tumor and no evidence of the primary tumor. The liver metastases were characterized by high proliferation index, immunoreactiviy for somatostatin receptor (SSTR)-1, 2, 3 and 5 and positive octreoscan. Urinary 5-hydroxyindolacetic acid, serum serotonin and chromogranin A were elevated. Slow release lanreotide (SR-LAN) therapy for 3 mo controlled clinical and biochemical signs of carcinoid tumor and caused a clear-cut reduction in the diameter of two liver metastases and disappearance of another lesion, with further reduction after 6 and 18 mo. We demonstrated a clear-cut long-lasting anti-proliferative effect of SR-LAN on liver metastases of occult carcinoid with high proliferation index and immunoreactivity for SSTR-1, 2, 3, and 5. Immuno-histochemistry for SSTRs could be a suitable method for the selection of patients with metastatic carcinoid that may benefit from SST analog therapy.
Keywords: Carcinoid, Somatostatin analogs, Somatostatin receptors
INTRODUCTION
Carcinoid tumors are the most frequent neuroendocrine tumors of the gastrointestinal (GI) tract[1]. Although initially believed to be mostly benign, these neoplasms have demonstrated different biological and clinical behavior and have recently been classified as either well-differentiated endocrine tumors or as well-differentiated or poorly differentiated endocrine carcinomas[2,3]. Carcinoid tumors, with the exception of those originating in the rectum, produce a variety of biologically active substances (most commonly serotonin and tachykinins), which may account for the carcinoid syndrome with flushing, diarrhea, bronchial constriction, and right heart disease[3,4].
Somatostatin (SST) is known to inhibit proliferation and secretion of normal and tumor endocrine cells expressing SST receptors (SSTRs). Five different SSTR subtypes have been identified and the anti-proliferative effects of SST have mainly been ascribed to SSTR1, SSTR2, and SSTR5 activation[5,6]. Long acting SST analogs (lanreotide, LAN, and octreotide, OCT) have been shown to be effective in controlling symptoms of carcinoid syndrome[4,7]. By contrast, few studies have demonstrated an anti-proliferative effect of these drugs on metastatic carcinoid tumors[8,9]. The clinical use of SST analogs is based on the expression of SSTRs in such tumors. This expression can be demonstrated by both in vitro and in vivo studies, the latter by SSTR scintigraphy[10]. The in vitro characterization of SSTR subtype expression has been carried out in only a limited number of studies, which have reported specific immunoreactivity for SSTR1 and SSTR2 in 86%, for SSTR3 in 71%, and for STTR5 in 83% of 36 carcinoid tumors[11].
In this report, we describe a patient with liver metastases of occult carcinoid tumor, in whom therapy with the slow release (SR)-LAN was associated not only with symptom improvement but also with sustained reduction in metastasis size. We also evaluated SSTR subtypes expression by immunohistochemistry and its correlation with in vivo SSTR scintigraphy.
CASE REPORT
A 72-year-old woman presented in 2002 with multiple liver lesions, incidentally discovered by abdominal ultrasonography (US) performed for gallstones follow-up. The patient reported a 2-year history of abdominal pain and intermittent diarrhea but did not complain of rash and flush episodes. Past medical history and family history were not remarkable and occurrence of multi-endocrine neoplasia type 1 (MEN1) was not documented. Physical examination was consistent with overweight (BMI = 29 kg/m2), mild hypertension (BP 145/90 mmHg) and normal heart rate. Serum electrolytes, complete blood count, liver and renal function were normal.
Abdominal CT showed multiple metastatic lesions at the VI hepatic segment (diameter 70 mm×60 mm) and at VII, V, and III segment (maximal diameters: 30, 10, and 10 mm, respectively) (Figure 1). US-guided liver biopsy demonstrated neoplastic cells, consistent with ‘metastases of neuroendocrine tumor’, with immunoreactivity for chromogranin A (CgA), neuron-specific enolase (NSE), and sinaptophysin. The Ki67 tumor proliferation index was 15%. In tissue specimens from liver metastases, immunohistochemical analysis was performed with specific antisera against SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5 (Gramsch Laboratories, Schwabhausen, Germany) at 1:200 dilution in PBS. Visualization was performed with avidin-biotin-peroxidase complex (ABC). The cell nuclei were counterstained with hematoxylin. All reagents were from Vector Laboratories (Burlingame, CA, USA). Strong membrane immunoreactivity was detected for STTR 1, 2, and 5; staining for SSTR3 was observed only in scattered cells, while no immunostaining was evident for SSTR 4 (Figure 2). Endocrinological work-out showed normal function of pituitary, thyroid and adrenocortical glands, and normal serum levels of calcium and parathyroid hormone. Urinary 5-hydroxyindolactetic acid (5-HIAA) and serum serotonin levels were elevated [14 mg/24 h (normal <5), and 248 ng/mL (normal <190), respectively] (Table 1). Plasma CgA levels were also increased (922 ng/mL; normal <98). By contrast, carcino-embryonic antigen and NSE plasma levels were normal. Echocardiography revealed no cardiac abnormalities. Whole body scanning with In-111 octreotide (Octreoscan) visualized the liver metastases (Figure 3), in the absence of other pathological uptake in the planar images obtained 24 and 48 h after injection. Esophagogastroduodenoscopy and barium study of the small bowel were normal. Colonoscopy showed a colon polyp, which on histological examination, was found to be a tubulovillous adenoma, with no evidence of primary carcinoid tumor either in the upper or in the lower GI tract. CT scan of the pelvis and thorax, bronchoscopy and bronchial brushing cytology were normal. Whole-body 18fluoro-2-deoxyglucose positron emission tomography, FDG-PET, confirmed the presence of a FDG-positive focus in the VII liver segment, without additional foci. At diagnosis, Karnofsky Performance Status Scale was 80% (100 = normal; 0 = dead), indicating the presence of some signs or symptoms of disease[12].
Figure 1.
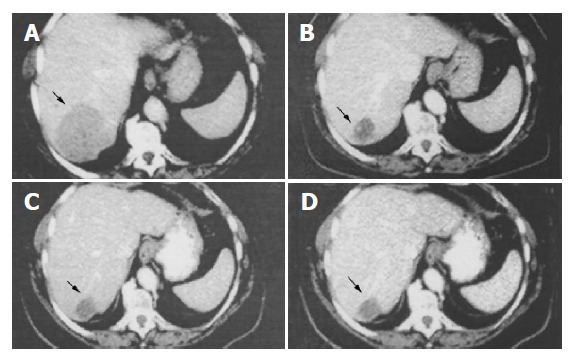
Abdominal CT scan obtained before initiation of SR-LAN therapy (A) showing the liver metastasis (black arrow) at the VI hepatic segment (diameter 70×60 mm). CT scan obtained after 3 (B), 6 (C) and 18 (D) mo of SR-LAN demonstrates a significant reduction in the size of liver metastasis.
Figure 2.
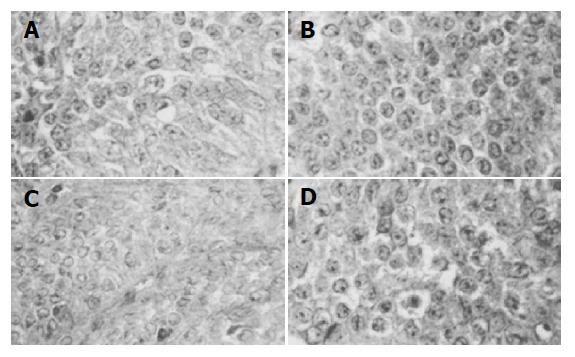
Most metastatic tumor cells in the liver demonstrate immunoreactivity for STTR1 (A), STTR2 (B), STTR3 (C), and STTR5 (D) on the cell membrane (×200).
Table 1.
Hormonal secretion before starting SR-LAN (0) and at 3, 6, 12 and 18 mo of therapy.
| 0 Before starting SR-LAN | 3 mo | 6 mo | 12 mo | 18 mo | |
| CgA (ng/mL) | 900.0 | 108.0 | 119.0 | 45.5 | 34.7 |
| NSE (ng/mL) | 6.9 | 5.0 | 5.8 | 6.9 | 6.3 |
| Serotonin (ng/mL) | 412 | 248 | 98 | 67 | 80 |
| 5-HIAA (mg/24 h) | 14.0 | 2.5 | 2.4 | 3.2 | 2.2 |
Figure 3.
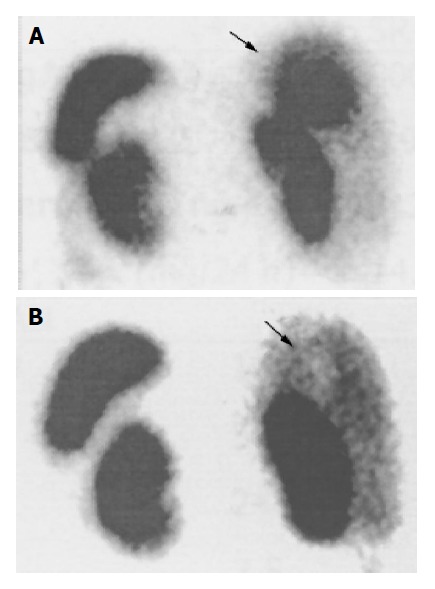
Liver metastasis (black arrow) visualized by whole body scanning with In-111 octreotide (octreoscan) before initiation of SR-LAN therapy (A). Octreoscan obtained after 12 mo of SR-LAN demonstrates a marked reduction in the liver uptake (B).
The patient was treated with 30 mg SR-LAN im every 14 d. Follow up-investigations, including complete blood- count, biochemical screening profile and evaluation of plasma CgA and serotonin, and urinary 5-HIAA levels were performed at 3, 6, 12 and 18 mo of therapy. CT scan was performed at 3, 6, and 18 mo and whole body octreoscan at 12 mo. The diarrhea was completely resolved after 1 mo of treatment. Serum serotonin and urinary 5 HIAA levels markedly decreased after 3 (serotonin 248 ng/mL; 5 HIAA, 2.5 mg/24 h) and 6 (serotonin 98 ng/mL; 5 HIAA, 2.4 mg/24 h) mo of therapy, remaining within normal range at 12 and 18 mo. Plasma CgA markedly decreased after 3 and 6 mo (108 and 119 ng/mL, respectively) and even more so after 12 and 18 mo of SR-LAN therapy (45.5 and 34.7 ng/mL, respectively) (Table 1). Abdominal CT showed reduction in the size of liver metastases (Figure 1) (maximal diameters: down to 35, 20, and 10 mm, respectively, at the V, VII, and V hepatic segment), and disappearance of the lesion at the III segment, after 3 mo of SR-LAN therapy. CT scan remained stable at 6 mo, and showed a further reduction in liver mass diameters (30 mm×28 mm for the lesion at the VI hepatic segment; 5 and 4 mm for the lesions at V and III segment, respectively) after 18 mo of therapy. Octreoscan confirmed the marked reduction in liver uptake without other sites of pathological accumulation of the tracer, at 12 mo after starting SR-LAN treatment (Figure 3). Serum electrolytes, complete blood- count, liver and renal function were still normal. No side- effects were reported, except for mild pain at the site of SR-LAN injection. Performance status improved with an increase in Karnofsky index at 3 mo (90%, indicating minor symptoms of disease), being unchanged after 6 and 18 mo of therapy.
DISCUSSION
In patients with liver metastatic carcinoid tumors therapeutic strategy includes several options: tumor debulking, embolization of the hepatic artery, treatment with long-acting SST analogs, interferon-α (INF α), combination of SST analogs and INF α, systemic chemotherapy, and liver transplantation[4,7]. SST analogs are of great value in the treatment and prevention of carcinoid crisis, resulting in disappearance or attenuation of symptoms in up to 60-85% of patients[10,13,14]. However, many patients may show desensitization, during treatment with LAN and OCT, within weeks to months[15]. Our patient showed persistent clinical and biochemical responsiveness to the treatment with 30 mg of SR-LAN administered every 2 wk for 18 mo, associated with an apparent reduction in liver metastases. The control of tumor growth and suppression of hormone hypersecretion ameliorated the quality of life in this patient with metastatic unresectable carcinoid tumor.
The anti-proliferative and pro-apoptotic effects of SST analogs have been demonstrated in several neuroendocrine tumors in vitro[6,16] and in vivo[17]. However, in humans the anti-proliferative effects have been quite variable and few clinical studies have demonstrated an antitumor activity of SST analogs in GI neuroendocrine tumors. Stable disease lasting from 3 mo up to 5 years has been achieved in 20-70% of patients, whereas only a partial response has been observed in <6% of patients[7,8,10,18]. In metastatic carcinoid tumors, 30 cases of tumor regression (defined as more than 50% reduction in tumor mass) have been reported in the literature[9] and complete regression of these tumors has rarely been shown[3,9,19,20].
Expression of SSTRs by neuroendocrine tumor may play an important role, both in detecting the tumor and in controlling the growth and hormone hypersecretion. Octreoscan can detect carcinoid and its metastases with a reported sensitivity of 82-95%[10], depending on tumor size and on SSTRs expression. In our patient, the staining pattern of SSTRs in specimens of liver biopsy was consistent with the presence of the SSTR subtypes 1, 2, 3 and 5, in keeping with previous evidence[11,21]. Moreover, the detection of metastatic lesions with octreoscan, predicted the biological and clinical response to therapy with SST analogs, as observed by other authors[3]. However, in some cases the results of SSTR scintigraphy are not consistent with those obtained through immunohistochemistry. In fact, the occurrence of a negative or low intensity uptake does not completely exclude possible tumor responsiveness to SST analogs and disease stabilization with this therapy[11,22].
The anti-proliferative effects of SST have been shown to be mediated via activation of SSTR1, 2 and 5[5]. LAN, as well as OCT, exhibits high affinity for SSTR2 and SSTR5 and low affinity for SSTR3[23]; in particular the expression of SSTR2 is required, if neuroendocrine tumors are to respond well to the currently used SST analogs[24,25]. SSTR3 is involved in apoptosis induction[5] and could explain the tumor shrinkage observed in selected patients treated with high doses of SST analogs[25,26]. Since different SSTR subtypes seem to be involved in the inhibition of hormone secretion, cell proliferation and apoptosis[5], SSTRs immunohistochemical analysis should be performed in all patients, with metastatic carcinoid lesions, in order to predict their clinical response to SST analog.
A slow tumor growth rate before SST analog treatment has been reported to predict a good response to OCT or LAN therapy in patients with neuroendocrine tumors[8]. It is known that elevated expression of nuclear antigen Ki-67 reflects high proliferative activity and may identify tumors with more aggressive biological behavior[2,27]. Furthermore, assessment of Ki-67 has been suggested to predict tumor growth stabilization with SST analog treatment[8]. In our patient, an elevated Ki-67 proliferation index was consistent with advanced disease, but was associated with a good responsiveness to the treatment with SR-LAN. Furthermore, our data suggest that an aggressive biological behavior does not exclude sensitivity to SST analogs.
Circulating tumor marker measurement may provide useful information for the follow-up and management of patients with carcinoid tumors[4]. In our patient, the reduction in urinary 5-HIAA and serum serotonin levels were consistent with the clinical response to treatment. Moreover, plasma CgA concentration significantly decreased during SR-LAN treatment concomitantly with the reduction in tumor mass, confirming that circulating CgA levels reflect the tumor mass bulk and may be a useful prognostic indicator in patients with metastatic carcinoid tumors[28].
In conclusion, a clear-cut, long-lasting anti-proliferative effect of SR-LAN was documented in a patient with liver metastases of occult carcinoid tumor, with high proliferation index and immunoreactivity for SSTR1, SSTR2, SSTR3, and SSTR5. Since individual SSTR expression pattern is important for tumor biology and growth behavior of neuroendocrine tumors, SSTR immunohistochemical analysis should be performed in clinical practice in order to identify patients with metastatic carcinoids that may benefit from treatment with specific SST analogs.
ACKNOWLEDGMENTS
Fondazione Cassa di Risparmio di Ferrara, Associazione Italiana Ricerca sul Cancro, Associazione Ferrarese dell’Ipertensione Arteriosa and IPSEN Pharmaceuticals.
Footnotes
Supported by the Grants From the Italian Ministry of University and Scientific and Technological Research (MIUR 2003069821-001; 60%, 2003)
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Rindi G, Capella C, Solcia E. Cell biology, clinicopathological profile, and classification of gastro-enteropancreatic endocrine tumors. J Mol Med (Berl) 1998;76:413–420. doi: 10.1007/s001090050233. [DOI] [PubMed] [Google Scholar]
- 3.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340:858–868. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 4.Oberg K. Diagnosis and treatment of carcinoid tumors. Expert Rev Anticancer Ther. 2003;3:863–877. doi: 10.1586/14737140.3.6.863. [DOI] [PubMed] [Google Scholar]
- 5.Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20:255–264. doi: 10.1385/ENDO:20:3:255. [DOI] [PubMed] [Google Scholar]
- 6.Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol. 2004;15:966–973. doi: 10.1093/annonc/mdh216. [DOI] [PubMed] [Google Scholar]
- 7.Zatelli MC, Tagliati F, Piccin D, Taylor JE, Culler MD, Bondanelli M, degli Uberti EC. Somatostatin receptor subtype 1-selective activation reduces cell growth and calcitonin secretion in a human medullary thyroid carcinoma cell line. Biochem Biophys Res Commun. 2002;297:828–834. doi: 10.1016/s0006-291x(02)02307-0. [DOI] [PubMed] [Google Scholar]
- 8.Aparicio T, Ducreux M, Baudin E, Sabourin JC, De Baere T, Mitry E, Schlumberger M, Rougier P. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur J Cancer. 2001;37:1014–1019. doi: 10.1016/s0959-8049(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 9.Leong WL, Pasieka JL. Regression of metastatic carcinoid tumors with octreotide therapy: two case reports and a review of the literature. J Surg Oncol. 2002;79:180–187. doi: 10.1002/jso.10062. [DOI] [PubMed] [Google Scholar]
- 10.de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10:451–458. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- 11.Kulaksiz H, Eissele R, Rössler D, Schulz S, Höllt V, Cetin Y, Arnold R. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50:52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45:2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Tomassetti P, Migliori M, Corinaldesi R, Gullo L. Treatment of gastroenteropancreatic neuroendocrine tumours with octreotide LAR. Aliment Pharmacol Ther. 2000;14:557–560. doi: 10.1046/j.1365-2036.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 14.Rohaizak M, Farndon JR. Use of octreotide and lanreotide in the treatment of symptomatic non-resectable carcinoid tumours. ANZ J Surg. 2002;72:635–638. doi: 10.1046/j.1445-2197.2002.02507.x. [DOI] [PubMed] [Google Scholar]
- 15.Corleto VD, Angeletti S, Schillaci O, Marignani M, Caratozzolo M, Panzuto F, Annibale B, Delle Fave G. Long-term octreotide treatment of metastatic carcinoid tumor. Ann Oncol. 2000;11:491–493. doi: 10.1023/a:1008398431246. [DOI] [PubMed] [Google Scholar]
- 16.Zatelli MC, Piccin D, Tagliati F, Ambrosio MR, Margutti A, Padovani R, Scanarini M, Culler MD, degli Uberti EC. Somatostatin receptor subtype 1 selective activation in human growth hormone (GH)- and prolactin (PRL)-secreting pituitary adenomas: effects on cell viability, GH, and PRL secretion. J Clin Endocrinol Metab. 2003;88:2797–2802. doi: 10.1210/jc.2002-021825. [DOI] [PubMed] [Google Scholar]
- 17.Borgström P, Hassan M, Wassberg E, Refai E, Jonsson C, Larsson SA, Jacobsson H, Kogner P. The somatostatin analogue octreotide inhibits neuroblastoma growth in vivo. Pediatr Res. 1999;46:328–332. doi: 10.1203/00006450-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 18.di Bartolomeo M, Bajetta E, Buzzoni R, Mariani L, Carnaghi C, Somma L, Zilembo N, di Leo A. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77:402–408. doi: 10.1002/(SICI)1097-0142(19960115)77:2<402::AID-CNCR25>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Todd JF, Meeran K. The tumour vanishes. Clin Endocrinol (Oxf) 2000;53:663–664. doi: 10.1046/j.1365-2265.2000.01109.x. [DOI] [PubMed] [Google Scholar]
- 20.Imtiaz KE, Monteith P, Khaleeli A. Complete histological regression of metastatic carcinoid tumour after treatment with octreotide. Clin Endocrinol (Oxf) 2000;53:755–758. doi: 10.1046/j.1365-2265.2000.01126.x. [DOI] [PubMed] [Google Scholar]
- 21.Kimura N, Pilichowska M, Date F, Kimura I, Schindler M. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin Cancer Res. 1999;5:3483–3487. [PubMed] [Google Scholar]
- 22.Zatelli MC, Piccin D, Bondanelli M, Tagliati F, De Carlo E, Culler MD, Uberti EC. An in vivo OctreoScan-negative adrenal pheochromocytoma expresses somatostatin receptors and responds to somatostatin analogs treatment in vitro. Horm Metab Res. 2003;35:349–354. doi: 10.1055/s-2003-41355. [DOI] [PubMed] [Google Scholar]
- 23.Arnold R, Wied M, Behr TH. Somatostatin analogues in the treatment of endocrine tumors of the gastrointestinal tract. Expert Opin Pharmacother. 2002;3:643–656. doi: 10.1517/14656566.3.6.643. [DOI] [PubMed] [Google Scholar]
- 24.Bertherat J, Tenenbaum F, Perlemoine K, Videau C, Alberini JL, Richard B, Dousset B, Bertagna X, Epelbaum J. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study. J Clin Endocrinol Metab. 2003;88:5353–5360. doi: 10.1210/jc.2002-021895. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson B, Renstrup J, Imam H, Oberg K. High-dose treatment with lanreotide of patients with advanced neuroendocrine gastrointestinal tumors: clinical and biological effects. Ann Oncol. 1997;8:1041–1044. doi: 10.1023/a:1008205415035. [DOI] [PubMed] [Google Scholar]
- 26.Imam H, Eriksson B, Lukinius A, Janson ET, Lindgren PG, Wilander E, Oberg K. Induction of apoptosis in neuroendocrine tumors of the digestive system during treatment with somatostatin analogs. Acta Oncol. 1997;36:607–614. doi: 10.3109/02841869709001323. [DOI] [PubMed] [Google Scholar]
- 27.Amarapurkar AD, Davies A, Ramage JK, Stangou AJ, Wight DG, Portmann BC. Proliferation of antigen MIB-1 in metastatic carcinoid tumours removed at liver transplantation: relevance to prognosis. Eur J Gastroenterol Hepatol. 2003;15:139–143. doi: 10.1097/00042737-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12 Suppl 2:S69–S72. doi: 10.1093/annonc/12.suppl_2.s69. [DOI] [PubMed] [Google Scholar]


