Abstract
AIM: To detect the presence of inducible nitric oxide synthase (iNOS), nitrotyrosine (NT) and apoptosis in gastric adenocarcinomas and their possible correlations with the clinicopathological characteristics and prognosis of gastric adenocarcinoma.
METHODS: Sixty-six specimens of gastric adenocarcinoma and corresponding adjacent normal gastric tissues were studied. Immunohistochemistry was employed to localize iNOS and NT protein and an immunohistochemical scoring system was used. The occurrence of apoptotic cell death (apoptotic index [AI]) was analyzed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) method.
RESULTS: Results showed that iNOS expression was detected at an intermediate or high level in 41 of 66 (62%) specimens of gastric adenocarcinoma. NT expression was 58%. Neither of them was found in the normal gastric tissues; there were significant positive correlations among iNOS expression, NT expression and AI. Many clinicopathologic characteristics of gastric adenocarcinoma, such as tumor size, depth of invasion, lymph node metastasis and TNM staging, were related to iNOS and NT expressions (P<0.05). In 66 surviving patients, the 5-year survival rate of 41 patients who had tumors with intermediate or high iNOS expressions and high AIs (4.09%; 19.96%) was significantly lower than that of 25 patients who had tumors with negative or low iNOS expressions and low AIs (0.79%; 47.14%) (P = 0.001). COX’s multivariate analysis revealed that the iNOS expression was identified as one of the significant independent prognostic factors predictive of a poor survival (relative risk [RR] = 2.69).
CONCLUSION: NO produced by iNOS may play a stronger role in promoting gastric adenocarcinoma growth than in suppressing its growth. iNOS and NT expressions by gastric adenocarcinoma may correlate with a poor survival.
Keywords: NOS, Nitrotyrosine, Gastric adenocarcinomas
INTRODUCTION
Gastric carcinoma is one of the most common malignant diseases in the world, and the first cause of gastrointestinal cancer-related mortality. The study conducted by Lala and Orucevic[1] showed that inducible nitric oxide synthase (iNOS) might play an important role in tumor growth, invasion, and metastasis. Thus, iNOS has been proposed to affect the clinical features of tumors[2-4]. Some tumors were reported to express iNOS, and the product of this enzymatic action (high output of NO), may induce peroxynitrite formation, resulting in a series of extensive oxidation reactions[5,6]. Peroxynitrite can cause damage to proteins, lipids, and nucleic acids[7], which may lead to cellular apoptosis and necrosis[8]. Peroxynitrite nitrates tyrosine residues of proteins, resulting in the production of nitrotyrosine (NT)[9]. Since this product is stable, the occurrence of NT in tissues has been measured as a marker indicative of formation and activity of the NO-derived oxidant peroxynitrite[10].
Previous studies indicate that effects of NO synthesized by iNOS can be either tumor suppressing or tumor promoting. The tumor suppressing role has been identified by the typical findings in NO-mediated apoptosis, including accumulation of tumor suppressor protein p53, caspase activation, chromatin condensation, and DNA fragmentation[11-13]. The tumor promoting role has been demonstrated by the evidence that NO produced by iNOS may promote tumor angiogenesis and blood flow in tumor neovasculature, and enhance tumor growth, invasion, and metastasis[1]. The conflicting dual roles of iNOS might work together to influence the clinical pathology and pathophysiology of tumors. However, when there is iNOS expression in gastric adenocarcinoma, the direction and condition in which the tumor may develop (promoted or suppressed) remains to be defined.
Our present study was designed to determine whether gastric adenocarcinoma cells expressed iNOS and NT, whether the distribution of NT in gastric adenocarcinoma was related to the apoptosis, and whether the presence of iNOS, NT and apoptosis correlated with clinical features of gastric adenocarcinoma and influenced patient’s prognosis.
MATERIALS AND METHODS
Specimens
Specimens of gastric adenocarcinoma and corresponding adjacent normal gastric tissues (at least 5 cm beyond the border of tumors, as control) were obtained from 66 surgically treated patients with gastric adenocarcinoma at the Department of Surgical Oncology, First Affiliated Hospital, China Medical University. Of them, 36 were males and 30 were females. The mean age was 57 years (range, 29-78 years). All the selected adjacent control gastric tissues were identified as normal by gross and microscope study. As to the grade of tumors, 36 cases were classified as poorly differentiated adenocarcinoma, of which 30 cases were well and moderately differentiated ones. None of the 66 patients received preoperative radiotherapy or chemotherapy, and all underwent curative surgical resection of the tumor along with regional lymph node dissection at least 5 years ago. Clinicopathologic characteristics of these patients were investigated based on the TNM classification of malignant tumors[14].
Resected specimens from the patients were fixed in 40 g/L formaldehyde and embedded in paraffin. Before immunohistochemical examination, hematoxylin-eosin staining was used to verify the pathology.
Immunohistochemical detections of iNOS and NT expressions
Immunohistochemical staining was performed by using the avidin-biotin-peroxidase complex (ABC) method. Mouse monoclonal antibodies against iNOS (diluted 1:100; Santa Cruz, Heidelberg, Germany) and mouse monoclonal anti-NT antibody (diluted 1:50; Zymed, Berlin, Germany) were used for immunostaining. The immunohistochemical staining steps were the same for both iNOS and NT. In brief, sections (4 μm) were fixed to the slides pretreated with polylysine (Sigma) and dried overnight at 50 °C. For immunohistochemistry the sections were deparaffinized, rehydrated in sequential alcohol baths, and washed with phosphate-buffered saline solution (PBS). The sections were boiled in 0.01 mol/L of citric acid buffer (pH 6.0) for 10 min, then cooled to room temperature and washed thrice with PBS for 9 min. The endogenous peroxidase activity was inactivated with 3% hydrogen peroxide in ethanol, and specific antibody binding was suppressed with 1.5% normal blocking serum in PBS. Sections were incubated in a humidified chamber for 2 h at room temperature. After intervening washes in PBS, sections were then incubated for 30 min with a biotin-conjugated secondary antibody. Goat anti-mouse immunoglobulin G (IgG; Santa Cruz) was used for anti-iNOS, and anti-NT antibodies. The avidin-biotin-immunoperoxidase complex (ABC staining system, Santa Cruz) was then applied. Peroxidase activity was visualized with diaminobenzidine (DAB; Boster, Wuhan, China). As controls, sections from all the samples were stained with the procedures described above, omitting the primary or secondary antibodies.
For iNOS and NT, weighted scores were computed, representing the percentage of positivity and intensity of gastric adenocarcinoma cells, as described previously[15]. The percentage of tumor cell positivity was categorized as follows: 0, <5%; 1, 5-25%; 2, 26-50%; 3, 51-75%, and 4, >75%. Staining intensity was graded as absent (0), weak (1+), medium (2+), or strong (3+). On the basis of weighted scores, expression was categorized as negative or low (0-4) and intermediate (5-8) or high (9-12). Before the study, specimens were scored, and representative sections displaying each of the four staining intensities were reviewed by two independent examiners.
Detection of apoptotic cells and bodies
Apoptotic gastric adenocarcinoma cells were detected by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) method. The ApoTag in situ detection kit (Boster, Wuhan, China) was used in this study. Briefly, paraffin sections were deparaffinized in xylene and rehydrated in graded ethanol. After being washed in Tri-buffer saline (TBS, pH 7.4), the sections were put into 3% H2O2 for 10 min, the tissues were then digested with proteinase K (20 μg/mL in TBS) at 37 °C for 10 min to enhance nuclear staining of apoptotic cells. The digestion was stopped with the sections washed in TBS. The sections were then treated with terminal transferase enzyme and digoxigenin-labeled nucleotides, and antidigoxigenin-peroxidase solution was applied afterwards. The color was developed with DAB, after which the sections were lightly counterstained with hematoxylin. To confirm the staining specificity of the TUNEL method, a positive control section was prepared. The substitution of equilibration buffer for TdT was used as a negative control.
The apoptotic index (AI) was expressed as the ratio of positively stained tumor cells and bodies to all tumor cells given as a percentage for each case. Positively stained tumor cells with morphological characteristics of apoptosis were identified by using standard criteria, including chromatin condensation, nucleolar disintegration, and formation of crescentic caps of condensed chromatin at the nuclear periphery[9,16]. To determine the AI, 10 representative areas without inflammation and necrosis but consisting of at least 1000 gastric adenocarcinoma cells were counted for each sample with a light microscope (×400 magnification). The results were assessed by two independent observers.
Statistical analysis
SPSS for Windows10.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Statistical significance was determined by χ2 test, Mann-Whitney U-test, and McNemar’s test. The null hypothesis for the McNemar’s test is that there is an agreement between the two parameters. Correlation between AI and NT expression was judged by the Spearman rank correlation test. Survival rates were calculated for the 66 patients by using the Kaplan-Meier method. Corrected survival rates were used, that is, only the deaths caused by gastric adenocarcinoma were taken as outcome events, and all the other deaths were considered as censored events. Log-rank test was used for univariate analysis to determine differences between curves. Multivariate analysis by Cox proportional hazards regression used only the variables that were significant in univariate analysis. P values less than 0.05 were considered statistically significant.
RESULTS
iNOS distribution
Positive iNOS staining was located in cytoplasm. iNOS was not only expressed in gastric adenocarcinoma cells, but also in inflammatory cells (Figure 1A). In contrast, in the normal gastric tissues, iNOS expression was barely detectable in epithelial and stromal cells (Figure 1C). Intermediate or high-level staining for iNOS was observed in 62.12% (41/66) of these specimens. The expression of iNOS was up-regulated with the advancement of the disease process. Gastric adenocarcinoma tissues, larger than 5 cm, expressed iNOS significantly stronger than smaller ones (P = 0.014). Intermediate or high iNOS expression was noted in 68% (28/41) of pT3/pT4 gastric cancers, but in only 32% (13/41) of pT1/pT2 gastric cancers (P = 0.039). Apart from that, TNM stages of the tumor affected iNOS expression, 75.61% (31/41) of stages III and IV tumors expressed an intermediate or high iNOS, whereas 24.39% (10/41) of stages I and II tumors showed an intermediate or high iNOS expression (P = 0.048). iNOS immunostaining rate in the patients with lymph node metastasis was higher than that in those without lymph node metastasis (P = 0.024). The other clinicopathologic characteristics, such as age, gender, degree of differentiation, Lauren’s classification and Borrmann classification did not show any significant relation to the expression of iNOS (Table 1).
Figure 1.
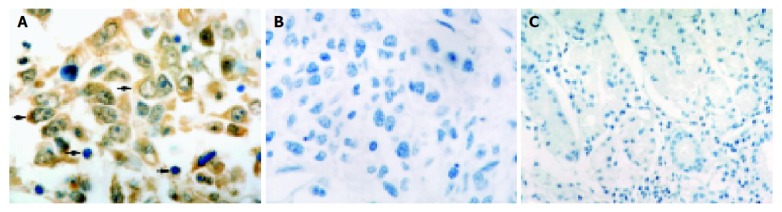
iNOS staining. A: iNOS staining detected in cytoplasms of tumor cells (thick arrow) and in inflammatory cells (thin arrows); B: Negative control; C: No positive immunohistostaining for iNOS in normal gastric tissue sections. Magnification A and B ×1000; C ×400.
Table 1.
iNOS expression and clinicopathologic features in gastric adenocarcinoma.
| Variable | Definition | Intermediate | Negative or high n (%) | P or low n (%) |
| Age (yr) | ≤59 yr | 22 (53.66) | 14 (56) | 0.853 |
| ≥60 yr | 19 (46.34) | 11 (44) | ||
| Gender | Female | 20 (48.78) | 10 (40) | 0.487 |
| Male | 21 (51.22) | 15 (60) | ||
| Tumor size | <5 cm | 12 (29.27) | 15 (60) | 0.014 |
| ≥5 cm | 29 (70.73) | 10 (40) | ||
| Borrmann classification | 0, 1, 2 | 20 (48.78) | 12 (48) | 0.951 |
| 3, 4 | 21 (51.22) | 13 (52) | ||
| Depth of invasion pT1, | 2 | 11 (26.83) | 13 (52) | 0.039 |
| pT3, 4 | 30 (73.17) | 12 (48) | ||
| Lymph node metastasis | Negative | 13 (31.71) | 15 (60) | 0.024 |
| Positive | 28 (68.29) | 10 (40) | ||
| Lauren's classification | Intestinal | 15 (36.59) | 12 (48) | 0.360 |
| Diffuse | 26 (63.41) | 13 (52) | ||
| Degree of differentiation | Well and moderate | 19 (46.34) | 11 (44) | 0.853 |
| Poor | 22 (53.66) | 14 (56) | ||
| TNM staging | III, IV | 10 (24.39) | 12 (48) | 0.048 |
| I, II | 31 (75.61) | 13 (52) |
Nitrotyrosine
Cytoplasms and nuclei of gastric adenocarcinoma cells were positive for NT staining, which was also detected in stromal cells around the tumor cells (Figure 2A). No NT staining was observed in the normal gastric tissues (Figure 2C). Thirty-eight of these sixty-six cases (57.58%) under study showed an intermediate or high expression for NT. The correlation between iNOS and NT expression within the same tumor specimen (P = 0.25) was found by McNemar’s test, suggesting that there might be an agreement between the two markers. All negative or low iNOS expression specimens were also negative or low for NT by immunohistochemistry. Only 3 of 41 intermediate or high iNOS expression specimens did not express NT. NT expression was related with the clinicopathologic characteristics of gastric adenocarcinoma. So was iNOS expression.
Figure 2.
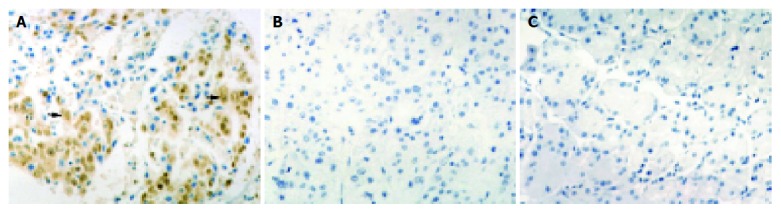
NT staining. A: NT staining detected in cytoplasms and nuclei of tumor cells (arrow); B: Negative control; C: No positive immunoreactivity in normal gastric tissue sections. Magnification ×400.
Apoptotic index (AI)
Almost all the positively stained cells and bodies were considered to be apoptotic cells corresponding morphologically to the standard criteria of apoptotic cells as defined previously. Nonspecific staining in necrotic foci showed a faint and diffuse staining and could be distinguished from the apoptotic nuclei by simple morphological examination (Figure 3A). The incidence of AI varied from 0% to 8.9%. The mean AI was 2.69% (s, 2.61) with a median of 2.09%. The mean AI in intermediate or high NT expression group was 4.09% (s, 2.57%), while it was significantly higher than the mean AI of 0.79% (s, 0.95%) observed in negative or low NT expression group by using the Mann-Whitney U test (P<0.001).
Figure 3.
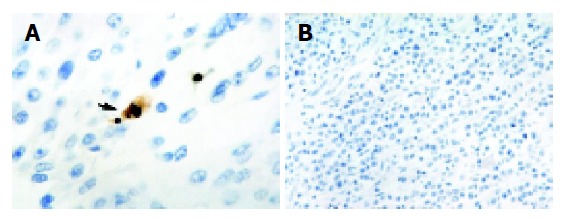
Apoptotic cells and bodies staining. A: An apoptotic body characterized by a pyknotic nucleus surrounded by shrunken cytoplasm and separated from the surrounding cells by a halo (arrow); B: Negative control. Magnification A ×1000; B ×400.
Correlations between AI and NT expressions
To examine whether NT expression corresponded to AI or not, the specimens were further stratified into different groups according to a comprehensive scoring system as described above. As AI was considered as a continuous variable, regression analysis with the Spearman rank correlation coefficient showed that AI had a significant direct correlation with NT expression scores (r = 0.718, P<0.001) (Figure 4).
Figure 4.
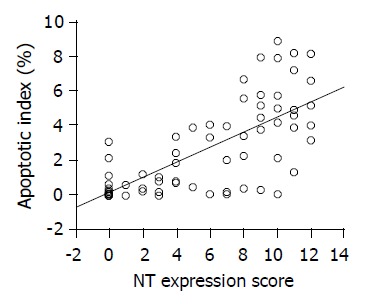
Significantly direct correlation between AI and NT expression observed by using the Spearman rank correlation coefficient (r = 0.718; P<0.001).
Survival analysis
After more than 5 years of follow up, 18 patients were alive at the time of this analysis, 4 patients died unrelated to gastric cancer, and the remaining 44 patients died of recurrent diseases. As a result, for the 66 subjects under study, the 5-year survival rate was 30.56%. The 5-year survival rate of the 41 patients in the intermediate or high iNOS expression group was 19.96%, which was significantly lower than 47.14%, the 5-year survival rate of the 25 patients in the negative or low iNOS expression group (P = 0.001). The 5-year survival curves in terms of iNOS expression are shown in Figure 5. Univariate analysis demonstrated that iNOS expression, depth of invasion, lymph node metastasis, TNM staging, tumor size, Lauren’s classification, Borrmann classification, and degree of differentiation were significant prognostic variables for survival (P = 0.001, P<0.001, P<0.001, P<0.001, P = 0.043, P = 0.032, P = 0.026, and P = 0.023, respectively; Table 2), while age and gender were not. However, Cox’s multivariate analysis revealed that iNOS expression, lymph node metastasis, TNM staging, and depth of invasion were significant independent prognostic factors (P = 0.012, P<0.001, P = 0.007, and P = 0.020, respectively; Table 2). The group with an intermediate or high iNOS expression and a high AI showed a poorer prognosis.
Figure 5.
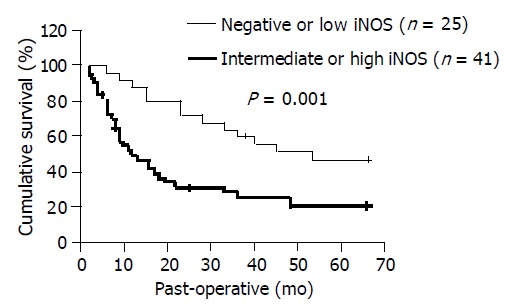
Survival of patients relative to iNOS status. Solid line: negative or low iNOS expression; dotted line: intermediate or high iNOS expression.
Table 2.
Univariate and multivariate regression analyses of prognostic indicators for long-term survival.
| Factor | Categories |
Univariate |
Multivariate |
|
| P (Log-rank test) | P (Wald test) | RR (95%CI) | ||
| iNOS expression | ≥5 scores | 0.001 | 0.012 | 2.69 (1.54.8) |
| ≤4 scores | ||||
| Depth of invasion | pT3, 4 | <0.001 | 0.020 | 4.11 (1.54.7) |
| pT1, 2 | ||||
| Lymph node metastasis | Positive | <0.001 | <0.001 | 5.77 (2.06.6) |
| Negative | ||||
| TNM staging | III, IV | <0.001 | 0.007 | 3.94 (1.47.2) |
| I, II | ||||
| Tumor size | ≥5 cm | 0.043 | NS | |
| <5 cm | ||||
| Lauren's classification | Diffuse | 0.032 | NS | |
| Intestinal | ||||
| Borrmann classification | 3, 4 | 0.026 | NS | |
| 0, 1, 2 | ||||
| Degree of differentiation | Poor | 0.023 | NS | |
| Well and moderate | ||||
| Age (yr) | ≤59 | 0.178 | ||
| ≥60 | ||||
| Gender | Female | 0.201 | ||
| Male | ||||
≥5 scores: intermediate or high iNOS expression; ≤4 scores: negative or low iNOS expression; RR: relative risk; CI: confidence interval; NS, not significant.
DISCUSSION
This study was undertaken to investigate the expression of iNOS and NT in gastric adenocarcinoma. Because NO is known to influence apoptosis, we also studied the extent of apoptosis in gastric adenocarcinoma.
Our results showed that normal gastric tissues did not express iNOS or NT, while the rate of iNOS expression in stages III and IV gastric adenocarcinoma was higher than that in stages I and II, revealing that expression of iNOS of gastric adenocarcinoma increased with the staging of cancer, which is in accordance with a previous study[17]. We also found that the expression of iNOS of gastric adenocarcinoma in patients with lymph node metastasis was higher than that in those without metastasis, suggesting that the significant increase of iNOS expression in gastric adenocarcinoma might contribute to lymph node metastasis of gastric cancer cells. Similarly, there were strong differences in iNOS immunostaining among the cases with different sizes of tumors or depths of invasion. But the degree of differentiation, Lauren’s classification, and Borrmann classification did not show any significant relation to the expression of iNOS. These results suggest that there is an up-regulation of iNOS positivity along with the biological aggressiveness of gastric cancer. The higher the iNOS expression of gastric cancer was, the more advanced was the gastric cancer, and the worse was the prognosis, suggesting the tumor-promoting role of iNOS, also shown in Koh’s[18] and Doi’s studies[19].
In our previous studies, frequent iNOS expression was also observed in many types of tumors such as colorectal, breast and bladder carcinomas and played an important role in tumor progression[20-22]. There is mounting evidence that NO acts as a carcinogen[23-26]. It was reported that elevated NO production could enhance the growth of some tumors through the suppression of antitumor immune responses[27,28]. Nitric oxide as a mediator of tumor vascularization might enhance tumor growth[29]. These observations suggest that NO may have some role in the growth, progression, maintenance, and/or metastasis of tumors.
Although this result is appealing, it has not been unanimously accepted. Some studies also revealed that iNOS activity was inversely correlated with tumor promotion, and some tumor cells lost their tumorigenic and metastatic abilities as a result of NO-mediated tumor-cell apoptosis[30,31]. Our study showed that there was a gradual increase in AI with a coexistent up-regulation of total iNOS in gastric cancer. The result suggested that NO produced by iNOS in gastric cancer and stromal cells could be an additional factor participating in the enhancement of gastric cancer cell apoptosis. Apoptosis mediated by iNOS may be one of the body’s defense mechanisms to restrict the growth of gastric adenocarcinoma, suggesting the tumor-suppressing role of iNOS.
It is thus obviously controversial as to the roles of iNOS in gastric clinicopathology as shown in our previous studies. However, the genetic constitution of cells may determine NO sensitivity or resistance. Lala et al[30] proposed that the genetic composition of tumor cells and the concentrations of NO in tumor-cell microenvironments were the main determinants of the role of NO. During clonal evolution of tumors in the presence of high NO concentrations, NO-sensitive cells might be deleted and NO-resistant cells might emerge owing to mutational events mediated by NO[30].
High AI of tumor cells in vivo, taken in isolation, might not necessarily indicate a favorable outcome. Our study showed that gastric carcinomas with intermediate or high iNOS and NT expressions had a higher AI and much poorer prognosis in the 5-year survival analysis. iNOS expression might have a higher predictive value (RR = 2.69) for determining the recurrence of gastric carcinoma patients following a curative resection. This indicates that in the process of gastric adenocarcinoma development the role of iNOS in enhancing tumor growth, invasiveness and metastasis may be stronger than that in promoting tumor cell apoptosis.
In conclusion, gastric adenocarcinomas can express iNOS and NT, and there is a correlation between NT and tumor apoptosis. Although tumors with a high AI may have a better prognosis, as noted by Ikeguchi[32] and Sugamura[33], tumor expression of iNOS and NT with a high AI is associated with a poor survival of gastric adenocarcinoma patients, which may result from the co-effects of a double-edged role of NO generated by iNOS. Further studies are necessary to investigate the precise mechanisms of this process.
ACKNOWLEDGMENTS
We gratefully acknowledge the help and support from Dr. Hui-Jun Cao, Beijing Institute of Radiation Medicine, Beijing and Dr. Fu-Jun Zao from West China Center of Medical Sciences, Sichuan University, Chengdu, China in statistical analysis of this study. We cordially thank Dr. Mei-Xian Wang, Department of Pathology, China Medical University and Mr. Chao Guan, Department of ENT, First Affiliated Hospital, China Medical University, Shenyang, China, for their help and valuable advice.
References
- 1.Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev. 1998;17:91–106. doi: 10.1023/a:1005960822365. [DOI] [PubMed] [Google Scholar]
- 2.Vickers SM, MacMillan-Crow LA, Green M, Ellis C, Thompson JA. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg. 1999;134:245–251. doi: 10.1001/archsurg.134.3.245. [DOI] [PubMed] [Google Scholar]
- 3.Sonoki T, Matsuzaki H, Nagasaki A, Hata H, Yoshida M, Matsuoka M, Kuribayashi N, Kimura T, Harada N, Takatsuki K, et al. Detection of inducible nitric oxide synthase (iNOS) mRNA by RT-PCR in ATL patients and HTLV-I infected cell lines: clinical features and apoptosis by NOS inhibitor. Leukemia. 1999;13:713–718. doi: 10.1038/sj.leu.2401398. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Morisaki T, Tsukahara Y, Uchiyama A, Matsunari Y, Mibu R, Tanaka M. Nitric oxide synthase expression and nitric oxide production in human colon carcinoma tissue. J Surg Oncol. 1999;70:222–229. doi: 10.1002/(sici)1096-9098(199904)70:4<222::aid-jso5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Davies MG, Fulton GJ, Hagen PO. Clinical biology of nitric oxide. Br J Surg. 1995;82:1598–1610. doi: 10.1002/bjs.1800821206. [DOI] [PubMed] [Google Scholar]
- 6.Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res. 1999;424:37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 7.Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci USA. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo S, Toyokuni S, Iwasa Y, Tanaka T, Onodera H, Hiai H, Imamura M. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free Radic Biol Med. 1999;27:401–410. doi: 10.1016/s0891-5849(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 9.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 11.Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997;11:443–448. doi: 10.1096/fasebj.11.6.9194524. [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler S, Zeiher AM. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 13.Brüne B, von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998;351:261–272. doi: 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari B, Coates PJ, Greenstein BD, Hall PA. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993;170:1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- 17.Rajnakova A, Moochhala S, Goh PM, Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. 2001;172:177–185. doi: 10.1016/s0304-3835(01)00645-0. [DOI] [PubMed] [Google Scholar]
- 18.Koh E, Noh SH, Lee YD, Lee HY, Han JW, Lee HW, Hong S. Differential expression of nitric oxide synthase in human stomach cancer. Cancer Lett. 1999;146:173–180. doi: 10.1016/s0304-3835(99)00265-7. [DOI] [PubMed] [Google Scholar]
- 19.Doi C, Noguchi Y, Marat D, Saito A, Fukuzawa K, Yoshikawa T, Tsuburaya A, Ito T. Expression of nitric oxide synthase in gastric cancer. Cancer Lett. 1999;144:161–167. doi: 10.1016/s0304-3835(99)00222-0. [DOI] [PubMed] [Google Scholar]
- 20.Yagihashi N, Kasajima H, Sugai S, Matsumoto K, Ebina Y, Morita T, Murakami T, Yagihashi S. Increased in situ expression of nitric oxide synthase in human colorectal cancer. Virchows Arch. 2000;436:109–114. doi: 10.1007/pl00008208. [DOI] [PubMed] [Google Scholar]
- 21.Vakkala M, Kahlos K, Lakari E, Pääkkö P, Kinnula V, Soini Y. Inducible nitric oxide synthase expression, apoptosis, and angiogenesis in in situ and invasive breast carcinomas. Clin Cancer Res. 2000;6:2408–2416. [PubMed] [Google Scholar]
- 22.Wolf H, Haeckel C, Roessner A. Inducible nitric oxide synthase expression in human urinary bladder cancer. Virchows Arch. 2000;437:662–666. doi: 10.1007/s004280000296. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamir S, deRojas-Walker T, Wishnok JS, Tannenbaum SR. DNA damage and genotoxicity by nitric oxide. Methods Enzymol. 1996;269:230–243. doi: 10.1016/s0076-6879(96)69025-9. [DOI] [PubMed] [Google Scholar]
- 25.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313(Pt 1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graziewicz M, Wink DA, Laval F. Nitric oxide inhibits DNA ligase activity: potential mechanisms for NO-mediated DNA damage. Carcinogenesis. 1996;17:2501–2505. doi: 10.1093/carcin/17.11.2501. [DOI] [PubMed] [Google Scholar]
- 27.Lejeune P, Lagadec P, Onier N, Pinard D, Ohshima H, Jeannin JF. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994;152:5077–5083. [PubMed] [Google Scholar]
- 28.Takahashi Y, Bucana CD, Akagi Y, Liu W, Cleary KR, Mai M, Ellis LM. Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin Cancer Res. 1998;4:429–434. [PubMed] [Google Scholar]
- 29.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lala PK. Significance of nitric oxide in carcinogenesis, tumor progression and cancer therapy. Cancer Metastasis Rev. 1998;17:1–6. doi: 10.1023/a:1005963400984. [DOI] [PubMed] [Google Scholar]
- 31.Juang SH, Xie K, Xu L, Shi Q, Wang Y, Yoneda J, Fidler IJ. Suppression of tumorigenicity and metastasis of human renal carcinoma cells by infection with retroviral vectors harboring the murine inducible nitric oxide synthase gene. Hum Gene Ther. 1998;9:845–854. doi: 10.1089/hum.1998.9.6-845. [DOI] [PubMed] [Google Scholar]
- 32.Ikeguchi M, Cai J, Yamane N, Maeta M, Kaibara N. Clinical significance of spontaneous apoptosis in advanced gastric adenocarcinoma. Cancer. 1999;85:2329–2335. [PubMed] [Google Scholar]
- 33.Sugamura K, Makino M, Kaibara N. Apoptosis as a prognostic factor in colorectal carcinoma. Surg Today. 1998;28:145–150. doi: 10.1007/s005950050096. [DOI] [PubMed] [Google Scholar]


