Abstract
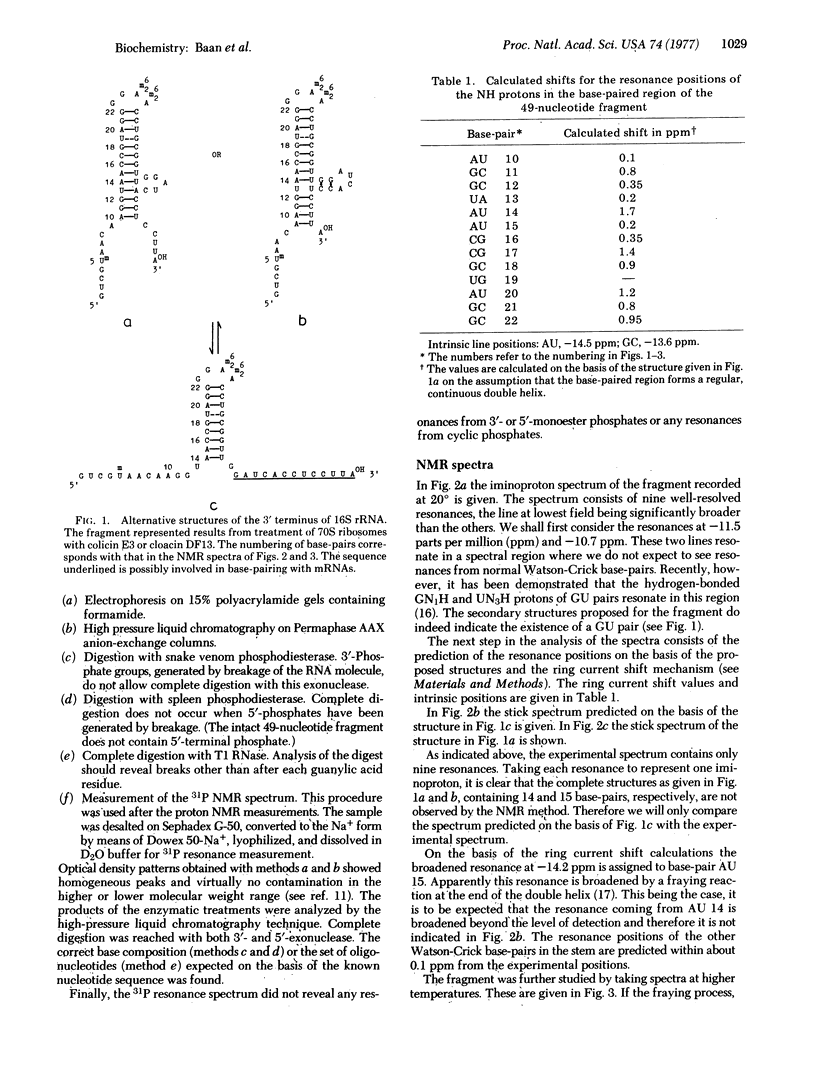
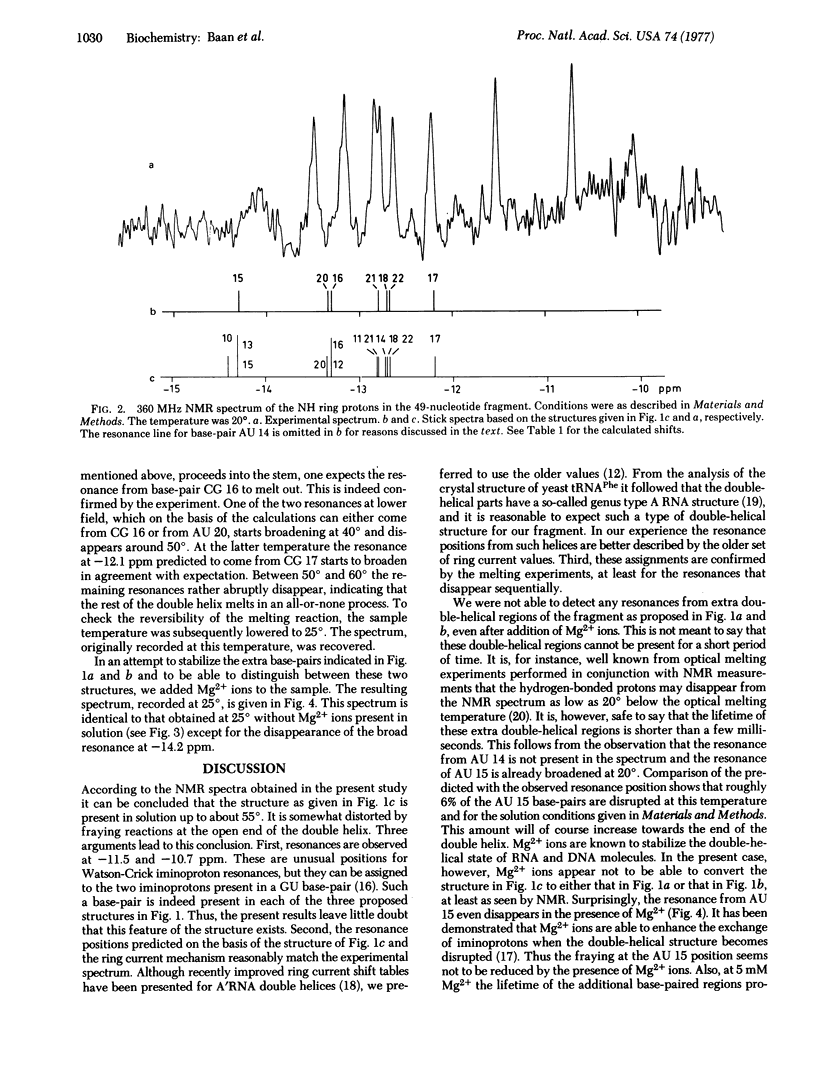
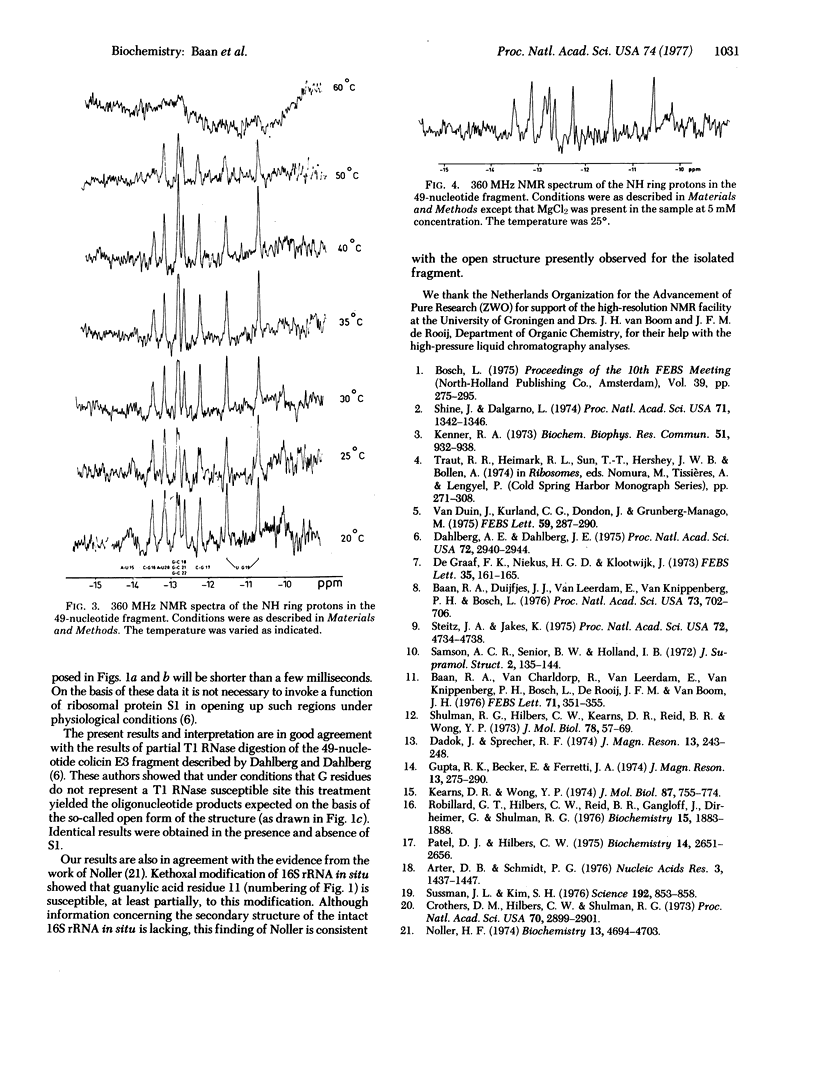
The 3' terminus of 16S rRNA has been implicated in the recognition of mRNA's by the ribosome. A fragment containing the 3'-terminal 49 nucleotides cleaved from the rRNA by cloacin DF13 was isolated in a pure form. The secondary structure of this fragment has been studied by measuring the high-resolution proton magnetic resonance spectra. The resonances observed at low field can be assigned to hydrogen-bonded iminoprotons of base-pairs present in the fragment. From the data we conclude that the rRNA fragment, under the conditions used, exists as a hairpin consisting of eight intramolecular base-pairs, the 3'-terminal dodecanucleotide being unpaired. The implications of these findings with respect to the function of the ribosomal protein S1 are discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arter D. B., Schmidt P. G. Ring current shielding effects in nucleic acid double helices. Nucleic Acids Res. 1976 Jun;3(6):1437–1447. doi: 10.1093/nar/3.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R. A., Duijfjes J. J., van Leerdam E., van Knippenberg P. H., Bosch L. Specific in situ cleavage of 16S ribosomal RNA of Escherichia coli interferes with the function of initiation factor IF-1. Proc Natl Acad Sci U S A. 1976 Mar;73(3):702–706. doi: 10.1073/pnas.73.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R. A., van Charldorp R., van Leerdam E., van Knippenberg P. H., Bosch L., de Rooij J. F., van Boom J. H. The 3'-terminus of 16 S ribosomal RNA of Escherichia coli. Isolation and purification of the terminal 49-nucleotide fragment at a milligram scale. FEBS Lett. 1976 Dec 1;71(2):351–355. doi: 10.1016/0014-5793(76)80968-4. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Hilbers C. W., Shulman R. G. Nuclear magnetic resonance study of hydrogen-bonded ring protons in Watson-Crick base pairs. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2899–2901. doi: 10.1073/pnas.70.10.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. R., Wong Y. P. Investigation of the secondary structure of Escherichia coli 5 S RNA by high-resolution nuclear magnetic resonance. J Mol Biol. 1974 Aug 25;87(4):755–774. doi: 10.1016/0022-2836(74)90083-7. [DOI] [PubMed] [Google Scholar]
- Kenner R. A. A protein-nucleic acid crosslink in 30S ribosomes. Biochem Biophys Res Commun. 1973 Apr 16;51(4):932–938. doi: 10.1016/0006-291x(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Topography of 16S RNA in 30S ribosomal subunits. Nucleotide sequences and location of sites of reaction with kethoxal. Biochemistry. 1974 Nov 5;13(23):4694–4703. doi: 10.1021/bi00720a003. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Hilbers C. W. Proton nuclear magnetic resonance investigations of fraying in double-stranded d-ApTpGpCpApT in H2O solution. Biochemistry. 1975 Jun 17;14(12):2651–2656. doi: 10.1021/bi00683a014. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Hilbers C. W., Reid B. R., Gangloff J., Dirheimer G., Shulman R. G. A study of secondary and tertiary solution structure of yeast tRNA(Asp) by nuclear magnetic resonance. Assignment of G.U ring NH and hydrogen-bonded base pair proton resonances. Biochemistry. 1976 May 4;15(9):1883–1888. doi: 10.1021/bi00654a014. [DOI] [PubMed] [Google Scholar]
- Samson A. C., Senior B. W., Holland I. B. The kinetics of colicin E3 induced fragmentation of Escherichia coli 16S ribosomal RNA in vivo. J Supramol Struct. 1972;1(2):135–144. doi: 10.1002/jss.400010206. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Hilbers C. W. Ring-current shifts in the 300 MHz nuclear magnetic resonance spectra of six purified transfer RNA molecules. J Mol Biol. 1973 Jun 25;78(1):57–69. doi: 10.1016/0022-2836(73)90428-2. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Kim S. Three-dimensional structure of a transfer rna in two crystal forms. Science. 1976 May 28;192(4242):853–858. doi: 10.1126/science.775636. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- van Duin J., Kurland C. G., Dondon J., Grunberg-Manago M. Near neighbors of IF3 bound to 30S ribosomal subunits. FEBS Lett. 1975 Nov 15;59(2):287–290. doi: 10.1016/0014-5793(75)80394-2. [DOI] [PubMed] [Google Scholar]



