Abstract
AIM: To study the effect of long-term ethanol consumption on jejunal lipase and disaccharidase (sucrase, maltase, and lactase) activities in rats and its gender difference.
METHODS: Age-matched male and female Wistar rats were fed control or ethanol-containing liquid diets for 12 wk following the Lieber-DeCarli model. According to both the plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities, 40 rats were divided into four groups as follows: male control group (MC), male ethanol group (ME), female control group (FC), and female ethanol group (FE).
RESULTS: After ethanol feeding for 12 wk, the results revealed that plasma AST and ALT activities of group ME were significantly increased by 58% and 92%, respectively, than those of group MC (P<0.05). Similarly, plasma AST and ALT activities of group FE were also significantly increased by 61% and 188%, respectively, than those of group FC (P<0.05). Fat accumulation was observed in both ethanol-treated groups, while fatty changes were more severe in group FE than those in group ME. The induction of hepatic microsomal cytochrome P450 2E1 (CYP2E1) was obviously seen in group ME and group FE, but was not detected in group MC and group FC. Jejunal lipase activity of group ME was significantly increased by 1.25-fold than that of group MC (P<0.05). In contrast to, sucrase, maltase, and lactase activities of group ME were significantly decreased by 63%, 62% and 67%, respectively, than those of group MC (P<0.05). Similarly, activities of these three enzymes of group FE were also significantly decreased by 43%, 46% and 52%, respectively, than those of group FC (P<0.05). There were no significant epithelial changes of the duodenal mucosa in any group.
CONCLUSION: Long-term ethanol consumption significantly can increase jejunal lipase and decrease jejunal disaccharidase activities in both male and female rats.
Keywords: Ethanol consumption, Jejunal lipase, Disaccharidase, Gender difference, Rats
INTRODUCTION
Alcohol research involving animals and humans has been dominated by investigations on males. However, recently increased effort has been undertaken to study the problems and mechanisms for alcohol intake in female animals. Now, it is well known that females are more susceptible to alcohol-induced liver injury than males[1,2]. Despite alcoholic liver disease, malnutrition sometimes occurs in alcoholics depending on duration and severity of alcohol abuse. Chronic alcohol consumption may result in malnutrition caused by poor dietary intake, altered digestion and absorption of nutrients. The reasons for impaired digestion and absorption of nutrients have been shown to be related to affect mucosal enzymes activities in small intestine in alcoholics[3].
It has been well known that the ethanol consumption disturbs the gastrointestinal function, which results in the poor digestion or absorption of nutrients. However, a limited number of studies have investigated the effects of chronic ethanol consumption on intestinal digestive enzyme activities such as disaccharidase[4] and lipase activities[5]. In addition, a few studies with the effect of gender difference on changes in the jejunal disaccharidase and lipase activities in rats after long-term ethanol administration. The aim of the present study was to determine whether a gender difference exists in alcohol-induced changes of jejunal lipase and disaccharidase activities.
MATERIALS AND METHODS
Animals and diets
Male and female Wistar rats purchased from the laboratory animal sources of the National Taiwan University College of Medicine were matched for age (6-wk old). Rats were fed with a standard laboratory diet and distilled water ad libitum, and housed in an air-conditioned room at 23±2 °C with 12 h of light per day. Experiments were started after the animals were allowed to adapt to the individual stainless-steel cages for 2 wk. According to both the plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities, 40 rats were divided into four groups. Two groups of male rats (10 rats/group) and two groups of female rats (10 rats/group) were fed with ethanol or isocaloric amounts of dextrose. Four groups were designed as follows: male control group (MC), male ethanol group (ME), female control group (FC), and female ethanol group (FE).
The composition of experimental diets was shown in previous study[6]. Rats in the ethanol group were fed liquid diets that provided 36% of total calories as ethanol, and rats in the control group received an isocaloric amount of the ethanol liquid diet in which the ethanol was replaced with maltose-dextrin. In order to avoid the evaporation of ethanol from liquid diets rapidly, rats were trained to eat at a fixed mealtime within a 2-h period.
Preparation of tissues
After 12 wk, all rats were anesthetized by carbon dioxide inhalation and killed. Blood was collected via the abdominal aorta. The liver was perfused with ice-cold physiological saline (9 g/L NaCl), and then removed for further analysis. Subsequently, the small intestine was immediately removed, and then washed in ice-cold physiological saline. The length of the small intestine was measured. The duodenal loops (about 1 cm) were removed, and then they were opened longitudinally and fixed in 40 g/L formaldehyde for histologic examination. The mucosal cells of jejunums (about 10 cm), where sucrase activity is most highly concentrated, were scraped off with a piece of glass and homogenized in ice-cold distilled water. The homogenates were centrifuged at 5000 r/min for 15 min at 4 °C. Supernatants were transferred into new Eppendorf tubes and stored at -80 °C for further analysis.
Measurement of ethanol concentration
The ethanol concentration was estimated colorimetrically with a commercial kit (Cat. No.: BA 106, Randox Laboratories, Antrum, UK) by following the product guide.
Measurements of plasma AST and ALT activities
At the beginning of experiment, blood samples were collected into heparin-containing tubes via the tail vein and centrifuged. Plasma AST and ALT activities were measured by spectroph-otometric methods with a latrozyme TA-LQ kit (Iatron Laboratories Inc., Tokyo, Japan).
Western blot analysis of hepatic microsomal CYP2E1 protein
Liver tissues were homogenized in 10 volumes of ice-cold buffer (0.25 mol/L sucrose, 10 mmol/L Tris–HCl, and 0.25 mmol/L phenylmethylsulfonyl fluoride, pH 7.4). The homogenates were centrifuged at 17000 g for 20 min at 4 °C. The supernatant was transferred to a new tube (Beckman 362305, Beckman Coulter Ltd, Bucks, UK) and centrifuged in a Beckman TLA 100.4 rotor at 105000 g for 60 min at 4 °C to separate the microsomes. The microsomal pellet was resuspended in 50 mmol/L potassium phosphate buffer containing 1 mmol/L EDTA and 1 mmol/L DTT (pH 7.4). Microsomal samples were aliquoted, frozen, and stored at -80 °C for further analysis. Thirty micrograms of microsomal protein and mid-range prestained MW standards (Mbiotech 20030, Mbiotech Inc., Seoul, Korea) were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) using Bio-Rad Minigel apparatus (Bio-Rad Laboratories, Hercules, CA). The stacking and resolving gels were composed of 4% and 10% polyacrylamide, respectively. After SDS-PAGE, the gels were electroblotted onto polyvinylidene difluoride membranes (Cat. No.: 10413096, Schleicher & Schuell BioScience, Keene, NH) using a semi-dry blotter (Hofer TE70, Semi-Dry Transfer Unit, Pharmacia, CA) at 100 mA for 2 h. The membranes were blocked for 2 h in PBS containing 1 mL/L Tween 20 (PBS-T) and 50 g/L non-fat dry milk at room temperature was then washed thrice with PBS-T. After blocking, the membranes were immunolabeled with the primary antibody in PBS-T for 2 h at room temperature and then washed thrice again with PBS-T. Bound antibodies were detected using a horseradish peroxidase-conjugated secondary antibody and visualized by chemiluminescence reagent using Western Lightning kit (Cat. No.: NEL104, NEL105, Perkin-Elmer Inc., Torrance, CA). To confirm equal protein loading in Western blot, the membranes were stripped with RestoreTM Western Blot Stripping Buffer (Cat. No.: 21059, Pierce Biotechnology Inc., Rockford, IL) according to the manufacturer’s directions and reprobed with actin antibody as an internal control. The antibodies used, and their dilutions, were as follows: mouse anti-rat CYP2E1 monoclonal antibody (Cat. No.: PM32, Oxford Biomedica Inc., San Diego, CA) 1:1000; mouse anti-actin monoclonal antibody (Cat. No.: MAB1501, Chemicon International Inc., Temecula, CA) 1:1000; goat anti-mouse IgG peroxidase conjugated antibody (Cat. No.: AP124P, Chemicon, International Inc., Temecula, CA) 1:5000.
Measurements of lipase and disaccharidase activities
Lipase activity was also measured with a commercial kit (Cat. No.: LI186, Randox Laboratories, Antrum, UK). Sucrase, maltase, and lactase activities were determined according to the method of Dahlqvist[7], as reported previously[4]. The specific disaccharidase activities were expressed as units/g protein. Total protein concentration was determined colorim-etrically by using a Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA).
Histologic examinations
As described in the preparation of tissue, all slices of liver and duodenal tissue were fixed in 40 g/L formaldehyde and embedded in paraffin for histologic examination. Sections were stained with hematoxylin-eosin, and examined under a light microscopy.
Statistical analysis
Values are expressed as mean±SE. To evaluate differences between the groups studied, two-way analysis of variance (ANOVA) with Fisher’s post hoc test was used. The SAS software (version 8.2, SAS Institute Inc., Cary, NC) was used to analyze all data. Differences were considered statistically significant when P<0.05.
RESULTS
Ethanol concentrations of liquid diet and blood
In order to avoid the evaporation of ethanol from liquid diets rapidly, rats were trained to eat at a fixed mealtime within a 2-h period. During this feeding time, the ethanol concentrations of liquid diets were maintained at the same level as the beginning point (Figure 1A). At 1 h after feeding, blood ethanol concentrations were highest (288±7 mg/dL), and they were still maintained above 200 mg/dL for at least 12 h (Figure 1B).
Figure 1.
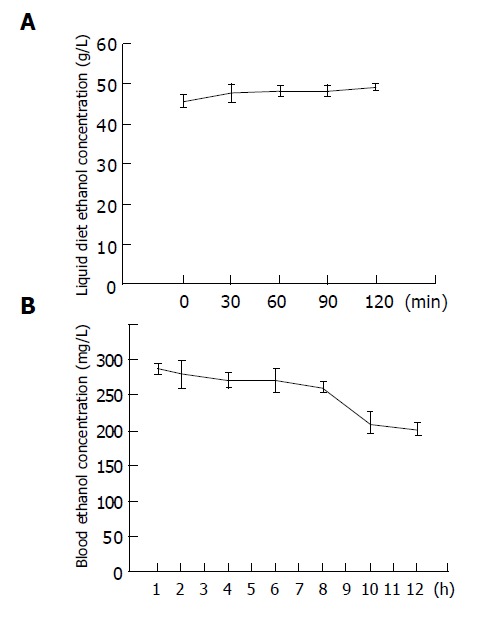
Changes in the ethanol concentrations of liquid diet (A) and blood (B) after ethanol feeding. Data are expressed as mean±SE (n = 10).
Energy intakes, body weights and liver weights
The energy intakes in the groups MC, ME, FC and FE were 155±6, 156±7, 153±7, and 155±7 kcal/(kg·d), respectively. And, the average alcohol intake in the groups ME and FE were 7.80±0.33 and 7.73±0.33 g/(kg·d), respectively, which was similar to that reported in other previous reports[6].
As shown in Table 1, there were no significant differences in initial body weight and liver weight between groups MC and ME. Neither were there significant differences between group FC and group FE. After ethanol feeding for 12 wk, the final body weight of group ME was significantly lower by 8% than that of group MC (P<0.05). However, there was no significant difference in final body weight between group FC and group FE. When compared to relative liver weight (% liver weight/body weight), the group ME and group FE was significantly increased by 12% and 16%, respectively, than that of group MC and group FC (P<0.05). In addition, significant gender differences were found for all measures. The initial and final body weight, liver weight, and relative liver weight in female rats were all significantly lower than those in age-matched male rats (P = 0.0113, P = 0.0001, P = 0.0001, and P = 0.0072, respectively). Moreover, significant ethanol differences were also found in final body weight and relative liver weight. The final body weight in ethanol-fed rats was significantly lower than that in control-fed rats (P = 0.0184); whereas, relative liver weight in ethanol-fed rats was significantly higher than that in control-fed rats (P = 0.0024).
Table 1.
Initial body weight, final body weight, liver weight, and relative liver weight in each group1-4.
| Groups | MC | ME | FC | FE |
ANOVA |
||
| Gender | Ethanol | Gender×ethanol | |||||
| Body weight(g) | |||||||
| Initial | 205±7 | 201±9 | 190±1 | 184±3 | 0.0113 | NS | NS |
| Final | 282±6 | 260±12 | 223±6 | 206±4 | 0.0001 | 0.0184 | NS |
| Liver weight (g) | |||||||
| Relative liver | 9.26±0.53 | 9.81±0.77 | 6.12±0.27 | 7.05±0.30 | 0.0001 | NS | NS |
| weight (%)5 | 3.34±0.15 | 3.73±0.17 | 2.88±0.09 | 3.33±0.10 | 0.0072 | 0.0024 | NS |
1Four groups were designed as follows: male control group (MC), male ethanol group (ME), female control group (FC), and female ethanol group (FE). 2Data are mean±SE (n = 10). 3Values in the same row with different superscripts are significantly different (P<0.05). 4Analyzed by two-way ANOVA; NS, no significant difference.
Relative liver weight = liver weight/body weight×100%.
Liver function and pathology
At the beginning of the experiment, there was no significant difference in plasma AST and ALT activities between male and female ethanol-fed rats and their corresponding controls (Table 2). However, significant gender differences were found for plasma AST and ALT activities. The initial plasma AST and ALT activities in female rats were all significantly lower than those in age-matched male rats (P = 0.0286 and P = 0.0003, respectively). After ethanol feeding for 12 wk, plasma AST and ALT activities of group ME were significantly increased by 58% and 92%, respectively, than those of group MC (P<0.05). The final AST and ALT activities of group FE were also significantly increased by 61% and 188%, respectively, than those of group FC (P<0.05). In addition, significant gender difference was found for final AST activity. The final AST activity in female rats was significantly higher than that in age-matched male rats (P = 0.0010). Moreover, significant ethanol differences were also found in final AST and ALT activities. The final AST and ALT activities in ethanol-fed rats were significantly higher than those in control-fed rats (P = 0.0001).
Table 2.
Plasma AST and ALT activities in each group1-4.
| Groups | MC | ME | FC | FE |
ANOVA |
||
| Gender | Ethanol | Gender×ethanol | |||||
| At the beginning of experiment | |||||||
| AST (U/L) | 86±3 | 87±2 | 81±2 | 82±2 | 0.0286 | NS | NS |
| ALT (U/L) | 28±1 | 28±1 | 24±1 | 24±1 | 0.0003 | NS | NS |
| At the termination of experiment | |||||||
| AST (U/L) | 95±10 | 150±12 | 126±8 | 203±16 | 0.0010 | 0.0001 | NS |
| ALT (U/L) | 38±03 | 073±05 | 033±1 | 095±13 | NS | 0.0001 | NS |
1-4Details as same as in Table 1.
Similarly, from the light micrograph of liver, histology was normal in control-fed rats (Figures 2A and 2C). In contrast, fat accumulation was observed in ethanol-treated groups (Figures 2B and 2D). Furthermore, fatty changes were severe and panlobular in ethanol-fed female rats (Figure 2D), but it was seen in pericentral areas in male ethanol-fed rats (Figure 2B).
Figure 2.
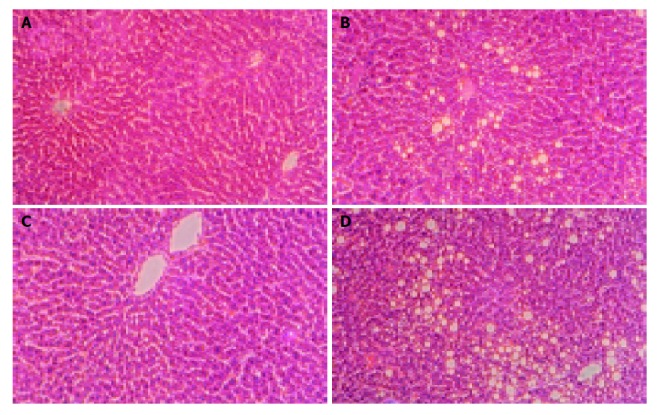
Histological examinations of specimens from the liver in each group. Livers from group MC (A), ME (B), FC (C), and FE (D) are shown. Sections of the liver from rats fed with control diet for 12 wk show normal histology with no evidence of pathologic change (A and C). Fat accumulation in female ethanol-fed rats was panlobular (D), but it was seen in pericentral areas in male ethanol-fed rats (B). (H&E stain, magnification: ×200).
Hepatic microsomal CYP2E1 expression
After ethanol feeding for 12 wk, the induction of hepatic microsomal CYP2E1 was obviously seen in groups ME and FE, but was not detected in groups MC and FC (Figure 3).
Figure 3.
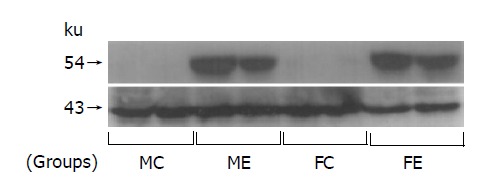
Representative Western blot of hepatic microsomal protein showing the induction of CYP2E1 due to chronic ethanol administration both in male and in female rats. Each lane represents an individual rat liver. The indicated apparent molecular masses of CYP2E1 and actin (internal control) were 54 and 43 ku, respectively.
Jejunal lipase and disaccharidase activities
As shown in Table 3, after ethanol feeding for 12 wk, jejunal lipase activity of group ME was significantly increased by 1.25-fold than that of group MC (P<0.05). However, there was no significant difference in jejunal lipase activity between groups FC and FE. Moreover, significant ethanol difference was also found in jejunal lipase activities. The specific activity of lipase in ethanol-fed rats was significantly increased than that in control-fed rats (P = 0.0074).
Table 3.
Jejunal lipase and disaccharidase activities in each group1-4.
| Groups | MC | ME | FC | FE |
ANOVA |
||
| Gender | Ethanol | Gender×ethanol | |||||
| Specific activities (U/g protein) | |||||||
| Lipase | 485±175 | 1093±264 | 458±123 | 891±137 | NS | 0.0074 | NS |
| Sucrase | 260±37 | 95±16 | 225±37 | 129±15 | NS | 0.0001 | NS |
| Maltase | 781±108 | 295±49 | 671±111 | 363±30 | NS | 0.0001 | NS |
| Lactase | 132±20 | 43±6 | 106±17 | 51±4 | NS | 0.0001 | NS |
1-4Details as same as in Table 1.
In contrast lipase, sucrase, maltase, and lactase activities of group ME were significantly decreased by 63%, 62% and 67%, respectively, than those of group MC (P<0.05). Similarly, sucrase, maltase, and lactase activities of group FE were also significantly decreased by 43%, 46% and 52%, respectively, than those of group FC (P<0.05). In addition, significant ethanol differences were found for sucrase, maltase, and lactase activities. The specific activities of these three enzymes in ethanol-fed rats were all significantly decreased than those in control-fed rats (P = 0.0001).
Morphology of duodenal mucosas
The duodenal morphology of rats was investigated by light microscopy. However, light microscopic examination of the duodenal mucosas revealed that there were no significant epithelial changes in any group (Figures 4A-4D).
Figure 4.
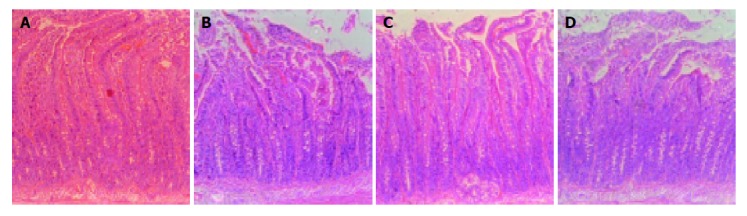
Histological examinations of specimens from the duodenum in each group (A: group MC; B: group ME; C: group FC; D: group FE. H&E stain, ×100).
DISCUSSION
This study investigated the effect of long-term ethanol consumption on jejunal lipase and disaccharidase (sucrase, maltase, and lactase) activities in rat and its gender difference.
A previous study has been reported that isocaloric substitution of carbohydrates by ethanol resulted in lower weight gain in spite of similar energy intakes[8]. This lower body weight gain has been attributed to induction of the microsomal ethanol-oxidizing system, increased sympathetic tone, and associated thermogenesis and/or enhanced ATP breakdown secondary to acetate production from ethanol[9]. In this study, the same effect of long-term ethanol intakes on body weight gain has been observed.
Both plasma AST and ALT are markers of liver damage as opposed to alcohol misuse. In this study, relative to gender-matched, paired-fed controls, chronic ethanol administration led to a significantly greater increase in both plasma AST and ALT activities in females than in males (Table 1). Furthermore, the female livers developed more severe alcohol-induced damage (Figure 2). It is well known that females are more susceptible to alcohol-induced liver damage than males. In an experimental study with adult male and female rats after 8 wk of ethanol administration according to the Lieber–DeCarli model as previously studied[6], ethanol-induced injury was more evident in female than in male livers[2]. The same effect has been seen in epidemiologic study[1], and also in one human study with alcoholics[10].
CYP2E1, a specific ethanol-inducible isoenzyme of P-450 has been isolated from hepatic microsomes of ethanol-treated rats and rabbits[11,12] and has also been confirmed in humans[13]. The induction of CYP2E1 contributes to the metabolic tolerance to ethanol that develops in chronic and heavy drinkers[14]. In the present study, we also confirm that long-term ethanol-fed male and female rat livers showed positive staining for CYP2E1, regardless of gender (Figure 3).
It has been reported that lipase activity was significantly greater in the intestinal lumen of the alcohol group than in the isocaloric glucose controls[5]. In another in vitro study, the author reported that chronic ethanol (50-300 mmol/L) administration to rat pancreatic cell line AR4-2J directly induced the expression and secretion of bile salt-dependent lipase. They also demonstrated that the increase of lipase activity was due to an enhanced biosynthesis of the enzyme consecutive to a major steady-state level of mRNA by ethanol[15]. In our study, the jejunal lipase activities were also significantly increased in ethanol-fed rats (Table 3).
In an experimental study with adult rats after 3 mo of ethanol consumption (300 mL/L in drinking water), the ileal maltase, lactase, and sucrase activities were decreased compared to control rats[4]. The same effect has been seen in previous in vivo[16] and in vitro studies[17], and also in one human study with alcoholic men[18]. However, there are still conflicting results in other experiments where no differences in disaccharidase activities were found[4,19]. Furthermore, an in vitro study showed that low concentrations of ethanol (1-3%) increased disaccharidase activities in the intestinal epithelial cell line[20]. The discrepancies in the disaccharidase activities might have been from differences in the ethanol dosage, duration (short term or long term), route of ethanol administration and individual nutritional status among the different studies[21]. In the present study, the jejunal disaccharidase activities were significantly decreased induced by long-term ethanol intakes not only in the male rats, but also in the female rats. We speculated that except for the direct reaction of ethanol intakes on the mucosal cell, the low carbohydrate (maltose-dextrin) contents might be one of the reasons which results in low disaccharidase activities.
It has been shown that chronic ethanol decreased jejunal cells, enterocyte height, and crypt cell population in rats after proximal jejunum resection[22]. But some other studies demonstrated normal histology by light microscopy in ethanol-fed rats[23] and in human study with chronic alcoholics. In the present study, there were no significant epithelial changes in any group. The low concentration of ethanol (5%) in Lieber-DeCarli model did not induce the histologic changes such as erosion and epithelial cell loss, although the digestive enzyme activities were influenced.
In conclusion, our results suggest that long-term ethanol consumption significantly increased jejunal lipase and decreased jejunal disaccharidase (sucrase, maltase, and lactase) activities in both male and female rats. Our results also show that chronic ethanol administration induced a greater susceptibility to liver damage in female rats than in male rats.
Footnotes
Supported by the Department of Health in Taiwan China, DOH93-TD-F-113-010
Co-first-authors: Chi-Chang Huang and Jiun-Rong Chen
Co-correspondents: Chi-Chang Huang
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 2.Colantoni A, Idilman R, De Maria N, La Paglia N, Belmonte J, Wezeman F, Emanuele N, Van Thiel DH, Kovacs EJ, Emanuele MA. Hepatic apoptosis and proliferation in male and female rats fed alcohol: role of cytokines. Alcohol Clin Exp Res. 2003;27:1184–1189. doi: 10.1097/01.ALC.0000075834.52279.F9. [DOI] [PubMed] [Google Scholar]
- 3.Bode JC, Knüppel H, Schwerk W, Bode C. Activities of cytoplasmic, mitochondrial and brush border enzymes in jejunal mucosa of chronic alcoholics. Z Gastroenterol. 1982;20:228–233. [PubMed] [Google Scholar]
- 4.Rodriguez-Castilla J, López-Nuevo M, Delgado MJ, Murillo ML, Carreras O. Changes in the ileal disaccharidase activities in rats after long-term ethanol feeding. Alcohol Alcohol. 1996;31:69–74. doi: 10.1093/oxfordjournals.alcalc.a008118. [DOI] [PubMed] [Google Scholar]
- 5.Mansbach CM. Effect of ethanol on intestinal lipid absorption in the rat. J Lipid Res. 1983;24:1310–1320. [PubMed] [Google Scholar]
- 6.Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol. 1994;233:585–594. doi: 10.1016/s0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- 7.Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968;22:99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- 8.Pirola RC, Lieber CS. Energy wastage in rats given drugs that induce microsomal enzymes. J Nutr. 1975;105:1544–1548. doi: 10.1093/jn/105.12.1544. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham CC, Spach PI. The effect of chronic ethanol consumption on the lipids in liver mitochondria. Ann N Y Acad Sci. 1987;492:181–192. doi: 10.1111/j.1749-6632.1987.tb48667.x. [DOI] [PubMed] [Google Scholar]
- 10.Gavaler JS. Sex-related differences in ethanol-induced liver disease: artifactual or real? Alcohol Clin Exp Res. 1982;6:186–196. doi: 10.1111/j.1530-0277.1982.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 11.Teschke R, Matsuzaki S, Ohnishi K, DeCarli LM, Lieber CS. Microsomal ethanol oxidizing system (MEOS): current status of its characterization and its role. Alcohol Clin Exp Res. 1977;1:7–15. doi: 10.1111/j.1530-0277.1977.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 12.Koop DR, Morgan ET, Tarr GE, Coon MJ. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982;257:8472–8480. [PubMed] [Google Scholar]
- 13.Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, Gonzalez FJ, Coon MJ, Gunsalus IC, Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993;12:1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- 14.Salaspuro MP, Lieber CS. Metabolic consequences of chronic alcohol consumption: attenuation of hepatic redox changes despite enhanced capacity to eliminate ethanol. Curr Alcohol. 1979;5:109–118. [PubMed] [Google Scholar]
- 15.Le Petit-Thevenin J, Pasqualini E, Nobili O, Vérine A, Lombardo D. Effects of ethanol on the expression and secretion of bile salt-dependent lipase by pancreatic AR4-2J cells. Biochim Biophys Acta. 1998;1408:44–54. doi: 10.1016/s0925-4439(98)00054-4. [DOI] [PubMed] [Google Scholar]
- 16.Barona E, Pirola RC, Leiber CS. Small intestinal damage and changes in cell population produced by ethanol ingestion in the rat. Gastroenterology. 1974;66:226–234. [PubMed] [Google Scholar]
- 17.Dinda PK, Hurst RO, Beck IT. Effect of ethanol on disaccharidases of hamster jejunal brush border membrane. Am J Physiol. 1979;237:E68–E76. doi: 10.1152/ajpendo.1979.237.1.E68. [DOI] [PubMed] [Google Scholar]
- 18.Perlow W, Baraona E, Lieber CS. Symptomatic intestinal disaccharidase deficiency in alcoholics. Gastroenterology. 1977;72:680–684. [PubMed] [Google Scholar]
- 19.Zarling EJ, Mobarhan S, Donahue PE. Effect of moderate prolonged ethanol ingestion on intestinal disaccharidase activity and histology. J Lab Clin Med. 1986;108:7–10. [PubMed] [Google Scholar]
- 20.Nano JL, Cefai D, Rampal P. Effects of ethanol on an intestinal epithelial cell line. Alcohol Clin Exp Res. 1990;14:32–37. doi: 10.1111/j.1530-0277.1990.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy DM, Nicholson JA, Kim YS. Intestinal enzyme adaptation to normal diets of different composition. Am J Physiol. 1980;239:G445–G451. doi: 10.1152/ajpgi.1980.239.6.G445. [DOI] [PubMed] [Google Scholar]
- 22.Zucoloto S, Braulio VB, Santos GC, Ramalho FS, Scandar MP, de Freitas O, de Oliveira JA. Effect of chronic ethanol consumption on the activities of residual small bowel brush-border enzymes after proximal jejunum resection in the rat. Alcohol Clin Exp Res. 1996;20:152–155. doi: 10.1111/j.1530-0277.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 23.Tamai H, Horie Y, Kato S, Yokoyama H, Ishii H. Long-term ethanol feeding enhances susceptibility of the liver to orally administered lipopolysaccharides in rats. Alcohol Clin Exp Res. 2002;26:75S–80S. doi: 10.1097/01.ALC.0000026981.32386.FD. [DOI] [PubMed] [Google Scholar]


