Abstract
AIM: To analyze possible relationships between CA IX/CA XII and pVHL expression in normal and neoplastic colorectal mucosa.
METHODS: Immunohistochemical staining of 42 tissue specimens obtained from 17 cancer patients was performed to evaluate the distribution and semi-quantitatively assess the levels of CA IX, CA XII and pVHL. VHL mRNAs from 14 fresh-frozen tumors was amplified by RT-PCR and subjected to sequencing. CA9 and CA12 mRNA levels were analyzed by semi-quantitative RT-PCR in comparison with VEGF as an indicator of hypoxia that uncouples the pVHL control.
RESULTS: Tumor tissues were associated with a borderline increase of CA IX staining signal and slight but significant decrease of CA XII immunoreactivity, whereas no association was found for pVHL. Sequence analysis of RT-PCR-amplified VHL mRNAs revealed no deletions/mutations, suggesting that they were VHL-competent. We did not observe any correlation between pVHL and CA IX/CA XII proteins as well as between VEGF and CA9 mRNAs, but the tumor-associated changes in mRNA levels of VEGF and CA12 showed a significant inverse relationship.
CONCLUSION: Our results indicate that CA9 and CA12 are regulated by different intratumoral factors and that lack of apparent relationship between the levels of CA IX/CA XII and pVHL cannot be fully assigned to uncoupling of negative regulatory function of pVHL by tumor hypoxia signified by induced VEGF transcription. The interplay between the functional pVHL and CA IX/CA XII in colorectal tumors seems rather complex and is not evident merely at the expression levels.
Keywords: Carbonic anhydrase, VHL, VEGF, Colorectal cancer
INTRODUCTION
The carbonic anhydrases (CAs) are an expanding family of zinc-containing enzymes, which classically participate in the maintenance of pH homeostasis in the human body, via catalysis of the reversible reaction: CO2 + H2O ⇔ HCO3- + H+ [1]. Immunohistochemical studies have shown that the transmembrane isoforms CA IX and XII, identified as tumor-associated proteins[2-6], are highly expressed in colorectal tissues and seem to be functionally related to development and/or spread of cancer[7,8]. Although the exact role of CA activity in carcinogenesis has not yet been established, these tumor-associated transmembrane isozymes IX and XII were proposed to acidify extracellular milieu surrounding the cancer cells and thus create a microenvironment conducive to tumor growth and spread[6,9]. This was recently proven for CA IX that can contribute to acidification of extracellular microenvironment under hypoxia[10]. The acidification can be reduced by CA IX-selective sulfonamides and deletion of the CA IX catalytic domain[10]. Moreover, several CA inhibitors may suppress the invasion capacity of some malignant cell lines[11]. One inhibitor of this type named Indisulam (E7070) has already been successful in phase II clinical trials for the treatment of colorectal cancer and non-small cell lung cancer[12]. In addition to enzyme-related function, CA IX appears to participate in tumor progression via disruption of E-cadherin-mediated cell-cell adhesion[13].
Previous studies have independently demonstrated two mechanisms, which control the expression of CA IX and CA XII. Regulation through hypoxia established in several recent studies clearly plays one of the major roles[14,15]. Transcription of CA9 and CA12 genes is induced by hypoxia in vitro and both proteins respond to tumor hypoxia in vivo, as judged from their perinecrotic intratumoral distribution[14,16-18]. CA9 is directly upregulated via binding of hypoxia-inducible factor (HIF) to hypoxia-response element (HRE) within the basal promoter, while the putative HRE of CA12 is far upstream of the promoter region and may rather work as an enhancer[14,19]. The other regulatory mechanism involves the protein product of the von Hippel-Lindau tumor suppressor gene (pVHL), which down-regulates the expression of CA IX and XII[6]. While both α- and β-domains of pVHL are required for the negative control of CA XII, the elongin-binding α-domain alone can effectively regulate CA IX (Figure 1). Recent studies have indicated that pVHL and HIF act within the interrelated pathways, which can regulate many proteins in addition to CA IX and XII[20,21]. A growing list of targets contains proteins implicated in angiogenesis, cell proliferation and survival, metabolism and other processes[22]. Karumanchi et al[23], reported that pVHL also regulates Cl-/HCO3- and Na+/H+ ion exchange activities, what may be of relevance for CA IX/CA XII, since some other CA isoforms were shown to directly interact with these ion exchangers in efficient transport metabolons[24,25].
Figure 1.
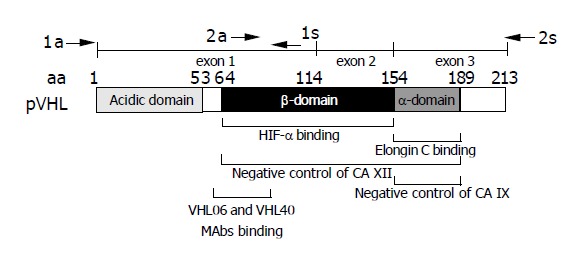
Schematic drawing of pVHL domain composition. Epitope region for the monoclonal antibodies VHL06 and VHL40 used in immunohistochemistry is shown below the scheme[30]. Positions of primers (a = antisense, s = sense) used for the amplification of two overlapping RT-PCR products are indicated above the exon map. Involvement of HIF-α-binding β-domain and elongin C-binding α-domain of pVHL in the negative regulation of CA XII and CA IX is illustrated according to data described by Ivanov et al[6].
It is well known that loss of VHL alleles or inactivating mutations in the VHL gene are involved in the pathogenesis of clear cell renal carcinoma[22]. This has naturally stimulated research into the target genes and proteins of pVHL, which might be expressed in renal cancer cells. It is notable, however, that CA IX and XII show a very distinct pattern of expression in normal versus malignant renal cells. CA IX is completely absent in the normal kidney, while it is highly expressed in renal tumors[26,27]. On the other hand, CA XII is produced by the epithelial cells of normal renal tubules and its expression remains at high level or is further increased in renal cancer[28]. Similar observation has been made in breast tissues, which do not belong to tissues affected by VHL disease[16]. These facts suggest that the response of CA IX and CA XII to pVHL/HIF pathways is modulated by different adverse regulatory factors in both renal and non-renal cells and may complicate the relationship between pVHL and CA IX/CA XII.
The present study focused on the colorectal tissues where CA IX, CA XII and pVHL are expressed in both normal and tumor tissues, but they were never compared, and the role of pVHL has remained unclear due to conflicting data on its status[7,8,29-32].
We evaluated the expression of pVHL in the normal and neoplastic colorectal mucosa and correlated its expression levels to those of CA IX and XII in the same specimens. Then we performed a sequence analysis of VHL mRNAs expressed in tumor tissues and after verifying the absence of mutations, assessed the mRNA levels of CA9 and CA12 in comparison with VEGF as an indicator of HIF activity that overrides the negative control of pVHL. We did not find any significant correlation between pVHL and CA IX/CA XII as well as between VEGF and CA9 mRNA expression. The only significant negative relationship was observed between tumor-associated changes in mRNA levels of VEGF and CA12. Our results indicate that the interplay between pVHL and CA IX/CA XII is more complex and cannot be observed merely at the expression levels.
MATERIALS AND METHODS
Antibodies
The production and characterization of the monoclonal antibodies to human CA IX and XII have been described earlier[33,34]. Both antibodies have been characterized by Western blots in which they have shown high isozyme specificity. Monoclonal antibodies against human pVHL have been characterized previously[30]. For the control purposes, all sections were immunostained using monoclonal mouse anti-human p53 antibody (CM-1) purchased from Novocastra Laboratories (Newcastle upon Tyne, UK).
Immunohistochemistry
The normal tissue samples from the large intestine and the corresponding benign and/or malignant neoplastic samples were obtained alongside routine histopathologic specimens collected at Oulu University Hospital (Oulu, Finland). The normal samples were excised separately 20 cm proximally from the tumor resection. The study was approved by the Ethics Committee of Oulu University Hospital and performed according to the guidelines of the Declaration of Helsinki.
The specimens of human colon (n = 32) and rectum (n = 9) were obtained from 17 patients. They consisted of 18 samples of histologically normal human colon or rectum and 23 colorectal lesions, including seven adenomas, and 16 adenocarcinomas. The adenomatous lesions included six tubular and one tubulovillous tumors. The grade of dysplasia was moderate in three lesions and grave in four lesions. The group of 16 malignant colorectal tumors consisted of 6 well differentiated, 9 moderately differentiated, and 1 poorly differentiated adenocarcinomas. There were three adenocarcinomas with a mucinous component. The primary lesions had been isolated from colon (n = 12), and rectum (n = 4). In addition, one metastasis of rectum carcinoma was included among tumor specimens.
The specimens were fixed either in Carnoy’s fluid (absolute ethanol++chloroform+glacial acetic acid 6:3:1) (staining for CA IX, XII, and pVHL) for 6 h or in 4% neutral-buffered formaldehyde (staining for p53) for 24-48 h. The samples were then dehydrated, embedded in paraffin in a vacuum oven at 58 °C, and sections of 5 μm were placed on gelatin-coated microscope slides. The CA isozymes and pVHL were immunostained by the biotin-streptavidin complex method, employing the following steps: (1) pre-treatment of the sections with undiluted cow colostral whey (Biotop Oy, Oulu, Finland) for 30 min and rinsing in PBS; (2) incubation for 1 h with primary antibodies diluted 1:10 (anti-CA IX hybridoma medium), 1:100 (anti-CA XII serum), and 50 µg/mL IgG (anti-VHL monoclonal antibody) in 1% BSA-PBS; (3) incubation for 1 h with biotinylated swine anti-rabbit IgG (Dakopatts, Glostrup, Denmark) or biotinylated goat anti-mouse IgG (Dakopatts) diluted 1:300 in 1% BSA-PBS; (4) incubation for 30 min with peroxidase-conjugated streptavidin (Dakopatts); (5) incubation for 2 min in DAB solution containing 9 mg 3,3’-diaminobenzidine tetrahydrochloride (Fluka, Buchs, Switzerland) in 15 mL PBS+5 μL 30% H2O2. The sections were washed thrice for 10 min in PBS after incubation steps 2 and 3, and four times for 5 min in PBS after step 4. All the incubations and washings were carried out at room temperature. For p53 immunostaining, formaldehyde-fixed sections were first boiled with 10 mmol/L citrate buffer pH 6 for 10 min, and the staining was further performed using CM-1 antibody as described earlier by Nuorva et al[35]. The stained sections were examined and photographed with Nikon Eclipse E600 (Tokyo, Japan) microscope.
The immunohistochemical results were semi-quantitatively assessed based on the percentage of the positive cells and on the intensity of the epithelial staining seen in a total field of a single section. The extent of staining (EXT) was scored by four of the investigators (AJK, JS, SP, and TJK) as 1, 2 and 3, when 1-10%, 11-50% or 51-100%, of the cells were stained, respectively. A negative score (0) was given to tissue sections that had no evidence of specific immunostaining. The intensity of staining (INT) was scored on a scale of 0-3 as follows: 0, no reaction; 1, weak reaction; 2, moderate reaction; 3, strong reaction. The relative protein expression index (on the scale 0-3) was calculated using the following formula: √ (EXT*INT) as described previously.
RNA extraction and reverse transcription
Extraction of total RNA from the snap-frozen colorectal specimens was done by disrupting the tissues in TRIzol solution (GIBCO, Life Technologies, Gaithersburg, MD). RNA was precipitated by ethanol, dissolved in water treated with diethylpyrocarbonate (DEPC; Sigma, St. Louis, MO) and reverse-transcribed with Mo-MuLV reverse transcriptase (Finnzymes OY, Finland) using random primers (500 μg/mL). Five microliters of RNase-free sterile water containing 1 μg of total RNA was added to a reaction mixture composed of dNTPs, each at 0.5 mmol/L concentration (Finnzymes), and reverse transcriptase buffer containing 6 mmol/L MgCl2, 40 mmol/L KCl, 1 mmol/L DTT, 0.1 mg/mL BSA and 50 mmol/L Tris-HCl, pH 8.3. The mixture in a final volume of 20 μL was heated for 10 min at 70 °C, cooled quickly on ice, supplemented with 200 U of reverse transcriptase, incubated for 1 h at 42 °C, heated for 15 min at 70 °C and stored at -80 °C until used.
PCR amplification
PCR reaction was performed with an automatic DNA thermal cycler using cDNA-specific primers for CA9, CA12, VHL, VEGF as well as for β2-microglobulin (β2M) that served as an internal standard. The nucleotide sequences of the primers, with relevant positions in parentheses, were as follows:
CA9: 5’-CCGAGCGACGCAGCCTTTGA-3’ (1167-1186)
5’-TAGTCGACTAGGCTCCAGTCTCGGCTACCT-3’ (1422-1400)
CA12: 5’-AAGGGAGTCATTTACAAGCCAG-3’ (1091-1112)
5’-AGGCCTGGCATGTTTGCAGATT-3’ (1270-1249)
VHL1: 5’-GTCCGGCCCGGGTGGTCTGG-3’ (41-60)
5’-AGCGTTGGGTAGGGCTGCGGC-3’ (374-354)
VHL2: 5’-TGCAATCGCAGTCCGCGCGTC-3’ (301-321)
5’-GAGATGAAACAGTGTAAGTTTCA-5’ (747-722)
VEGF: 5’-GGGATCCTGAACTTTCTGCTGTCTT-GGGTG-3’
5’-TGAATTCCCTCACCGCCTCGGCTTGTCACA-3’
ß2M: 5’-CATCCAGCGTACTCCAAAGA-3’ (860-879)
5’-GACAAGTCTGAATGCTCCAC-3’ (1024-1005)
Reaction mixture was composed of: 1/40 of cDNA template obtained from 1 μg of RNA, 15 pmoL of each upstream and downstream primers for each analyzed gene and β2M, 0.2 mmoL of each dNTP (Amersham Biosciences), 1 U of Taq DNA polymerase (Boehringer, Mannheim, Germany) and PCR buffer (10 mmol/L Tris-HCl pH 8.3, 50 mmol/L KCl and 1.5 mmol/L MgCl2) in a total volume of 25 μL. Following an initial denaturation at 95 °C for 1 min, the amplification program was set as follows: denaturation at 95 °C for 20 s, annealing at 65 °C for 30 s, and extension at 72 °C during 40 s for a total of 30 cycles, and finally 5 min at 72 °C. In addition to the samples studied, negative and positive controls were systematically processed in parallel. VHL-specific PCR products were separated on agarose gel, eluted, and directly sequenced on automatic DNA sequencer. Sequence data were analyzed for the presence of mutations using BLAST software.
Semiquantitation of RT-PCR products
Twenty microliters of RT-PCR products were run on a 1.8% agarose gel. Quantification of PCR products was done using MCID M4 image analysis software (Imaging Research Inc., St. Catharines, Ontario, Canada). For semi-quantitative assessment, the amount of specific PCR product was expressed as a relative proportion of co-amplified internal standard (ß2M).
Statistical analysis
Association between the levels of proteins/transcripts in colorectal tissues was evaluated by χ2 test of independence using data obtained in parallel analyses of the same specimens. Mann-Whitney rank test was used to compare the expression levels of individual markers in normal vs tumor specimens. Value of P≤0.05 was considered as significant and P≤0.01 as highly significant in both tests.
RESULTS
Expression of CA IX, CA XII and pVHL
As a first step toward exploring a possible relationship between CA IX/CA XII and pVHL, we performed an immunohistochemical staining of colorectal tissue specimens with the corresponding specific antibodies including the antibody against p53 that was used as a positive control for pathologic lesions. Based on both extent and intensity of the staining signal, we derived staining indices whose values represented semi-quantitative assessment of the protein expression levels. The data obtained for each protein were divided into two groups and analyzed by Mann-Whitney test to allow for comparison of expression in the normal versus tumor tissues (Figure 2). Expression of p53 was significantly higher in the pathologic tissues than in the normal counterparts (P<0.0001) and also CA IX showed an association with tumors at the borderline significance (P = 0.0516). On the other hand, CA XII displayed small, but highly significant decline of expression in tumors (P = 0.0002). Finally, no significant difference in normal vs tumor-related levels was observed for pVHL (P = 0.494). As evident from the graphical illustration, ranges of staining indices for all examined proteins were broader in tumor specimens compared to normal tissues indicating higher variability of their expression in pathologic conditions.
Figure 2.
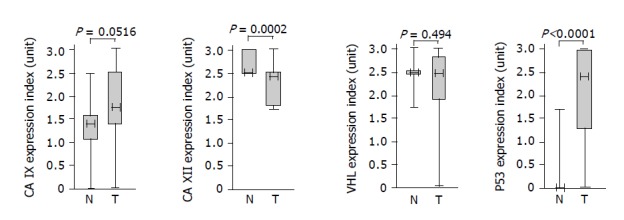
Associations of the proteins with colorectal tumors based on semi-quantitative immunohistochemical assessment and analysis of the data using the Mann-Whitney rank test. Each box indicates the range of staining indices from the 25% to the 75% quantile, the horizontal line denotes the median and the whiskers above and below the box show the highest and lowest index. Values of significance are given above the graphs.
Figures 3-5 show examples of pVHL, CA IX, and CA XII immunohistochemical staining patterns in the mucosa of the normal colon and in the colorectal lesions. Almost all normal enterocytes exhibit cytoplasmic staining of pVHL (Figure 3B). Similarly, most adenomas and malignant lesions were positive for pVHL (Figures 4, 5B and 5F). CA IX expression in normal mucosa was focal and confined to membranes of cryptal epithelial cells (Figure 3C), while in tumors it considerably varied among adjacent areas having different histopathologic characteristics (Figures 4, 5C and 5G). Focal CA IX expression with a strong staining intensity was observed in desmoplastic areas of the three mucinous adenocarcinomas (Figure 4C). In contrast, CA XII immunostaining was diffuse in the normal mucosa (Figure 3D), with the strongest signal in the most luminal part, and was slightly diminished in the neoplastic lesions (Figures 4, 5D and 5H). All these findings were in accord with the earlier observations[7,8,29,30].
Figure 3.
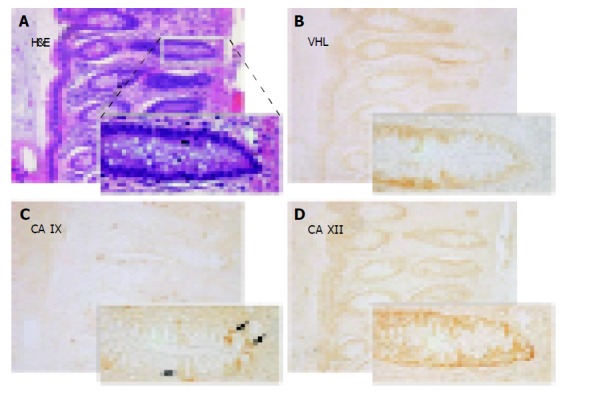
Immunohistochemical detection of pVHL (B), CA IX (C) and CA XII (D) in serial sections from the normal colon with details shown on magnified areas in the left corners. Arrows in panel C designate a focal membrane signal specific for CA IX. A shows hematoxylin-eosin (H&E) staining of a parallel section. Original magnification, ×100.
Figure 5.
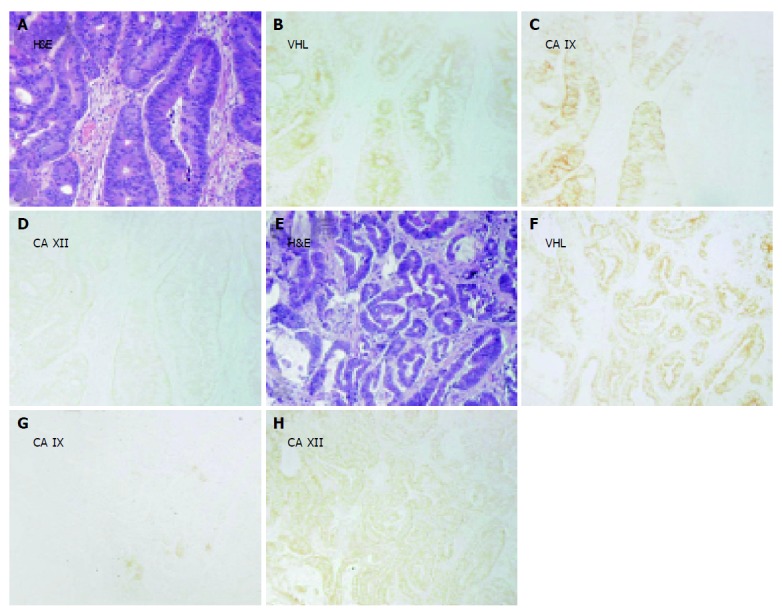
Examples of immunohistochemical staining of pVHL, CA IX and CA XII in parallel sections of adenocarcinoma (grade II) with (A-D) or without (E-H) mucinous component. One crypt remains negative for CA IX (C). Corresponding H&E staining is shown in panels A and E. Original magnifications, ×100.
Figure 4.
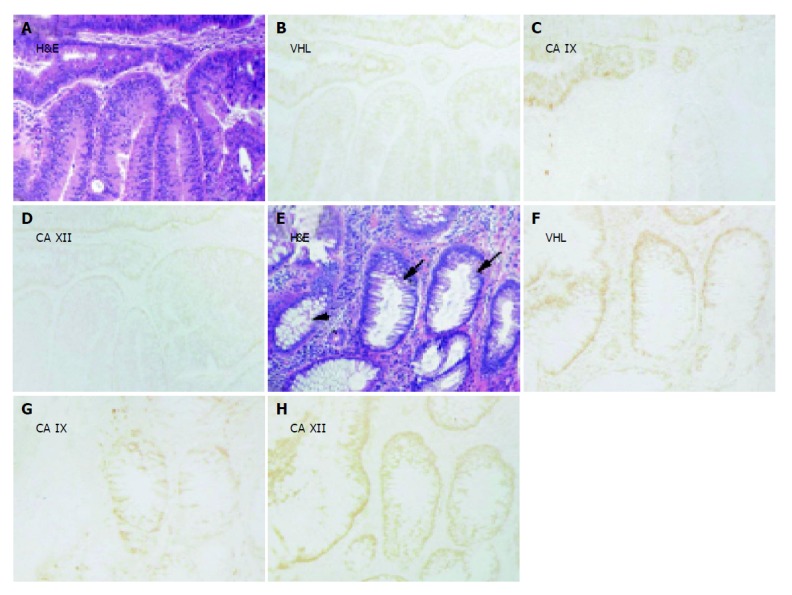
Immunohistochemical staining of pVHL, CA IX and CA XII in parallel sections of (B-D) moderate (lower part) and grave (upper part) adenomas and of (F-H) transitional epithelium (small arrows) located between histologically normal region (arrowhead) and grade I adenocarcinoma (not shown). Corresponding H&E staining is shown in panels A and E. Original magnifications, ×100.
To get better insight into changes in expression levels of the studied proteins that had happened in each patient during the development of neoplasia, we subtracted the value obtained in normal tissue from that coming from tumor of the same person. In those cases, where more than one pathologic specimen was available, we used a mean value for the calculation of the difference in order to deal with a paired data and permit further comparisons (Figure 6). Our analysis revealed that expression of p53 in tumors was never lower than in corresponding normal tissues. The opposite was true for CA XII that showed either no change in 3 cases or decrease in 14 cases. Interestingly, this decrease could be attributed to intensity only, while the extent of expression remained the same in all but one patient (not shown). Level of CA IX was increased in 10 tumors, unchanged in 2 tumors and decreased in 5 cases, whereas the differences in pVHL were allotted more evenly with increase in 5 cases, no change in 4 cases and decrease in 6 cases. No relationship among CA IX, CA XII and pVHL proteins has become apparent when looking for their simultaneous changes in individual patients.
Figure 6.
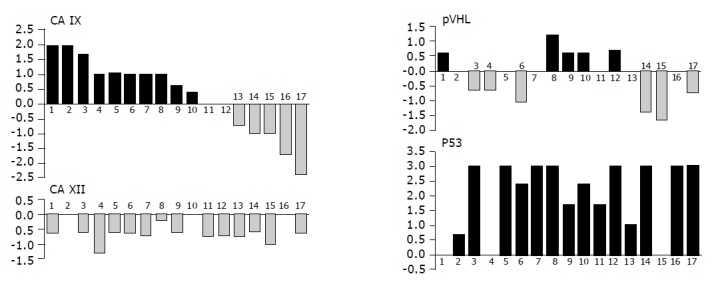
Graphical illustration of the tumor-associated differences in expression of pVHL, CA IX, CA XII and p53 in individual patients analyzed by immunohistochemistry (the same person is designated by the same number in all graphs). Values of staining indices obtained in normal tissues were subtracted from those obtained in corresponding pathological lesions from the same person (mean value was used when more tumor specimens were available from one patient). Resulting data were shown in histograms on compatible scales demonstrating the range of differences related to each marker and allowing for their visual comparisons. There were no relationships found between the studied proteins.
Sequence analysis of VHL RT PCR products
Considerably diminished or lost positivity for pVHL in several carcinomas prompted us to examine VHL gene expression at the level of mRNA by reverse transcription PCR using pairs of primers designed to generate two overlapping products (Figure 1). The RNA was isolated from the snap frozen tissues of 14 patients. Both the expected VHL-specific amplification products were obtained from all normal and tumor specimens including those tissues that showed decreased or lost staining of pVHL in immunohistochemistry (not shown). The antibodies VHL06 and VHL40 used for the pVHL detection were directed against the epitope in amino acids 60-89 within a β domain and could recognize the wild type as well as the C-terminally mutated protein[30]. To look into the status of the VHL mRNAs in tissues involved in this study, all tumor-derived RT PCR amplicons were subjected to direct sequencing. Sequence analyses confirmed the absence of deletions as well as inactivating mutations in this series of colorectal carcinomas indicating that the corresponding tumors were VHL competent.
Expression of CA9, CA12 and VEGF mRNA
The wild type pVHL has been previously shown to work as a negative regulator of transcription of the genes coding for carbonic anhydrases IX and XII in renal carcinoma cells[6]. This function of pVHL was abolished in other types of tumors under hypoxic conditions when both CA9 and CA12 mRNAs were induced at the level of transcription[14]. For CA IX, this transcriptional activation was proven to occur through HIF transcription factor whose alpha subunit escapes degradation by pVHL and binds to HRE element in the CA9 promoter[14]. HIF co-ordinately induces a number of targets including VEGF[21] and significant correlation was found between the expression of HIF-1α and VEGF in colorectal carcinoma[36]. Therefore, we decided to follow the level of VEGF mRNA as an indicator of HIF activity in our collection of tissues from 14 persons, for which RNA was available. Each reverse transcribed cDNA was used as a template for three parallel PCR reactions to semi-quantitatively assess the levels of VEGF, CA9 and CA12 mRNAs. Similarly as described above for immunohistochemistry, values obtained for normal tissues were subtracted from the tumor-related values to get an idea about changes associated with neoplasia in individual persons (Figure 7). In accord with IHC analysis, majority of tumor specimens (nine cases) displayed increased levels of CA9 mRNA. Expression of CA12 was unchanged or decreased in more than half of tumors, but six specimens showed increased CA12 levels (Figure 7). VEGF mRNA level was increased in 10 cases and unchanged or somewhat decreased in 4 cases. Interestingly, majority of tumors showing increased VEGF mRNA levels simultaneously displayed lower or equal CA12 expression and vice versa. Indeed, χ2 analysis performed with the data grouped in two categories, namely increase (+) vs no increase (0/-), revealed a highly significant negative association between VEGF and CA12 (P = 0.0063). No association could be found between CA12 and CA9 (P = 0.3340) as well as between CA9 and VEGF (P = 0.4805). Although obtained in a small cohort of colorectal tumor patients, this finding may suggest that CA9 and CA12 are regulated by different intratumoral factors and that lack of apparent relationship between the levels of CA IX/CA XII and pVHL cannot be fully assigned to uncoupling of pVHL negative regulatory function by tumor hypoxia signified by induced VEGF transcription.
Figure 7.
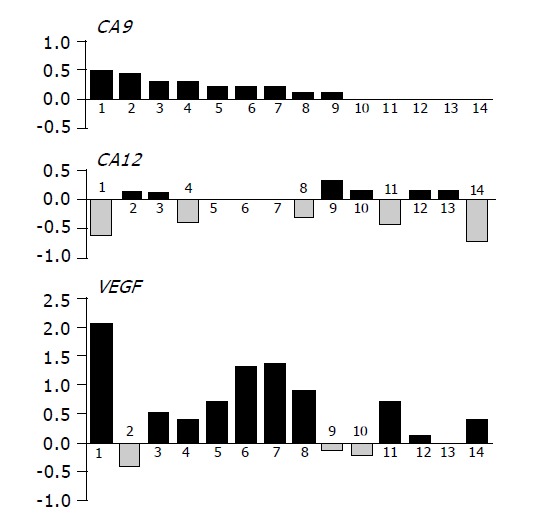
Graphical illustration of the tumor-associated differences in mRNA levels of CA9, CA12 and VEGF in individual patients analyzed by RT-PCR. Values representing relative amounts of mRNAs expressed in normal tissues were subtracted from those obtained in paired pathological lesions. Resulting data were drawn in histograms on compatible scales allowing for their comparisons. Highly significant inverse relationship was found between CA12 and VEGF by χ2 analysis (P = 0.0063).
DISCUSSION
Von Hippel-Lindau protein (pVHL) executes its tumor suppressor function principally via down-regulation of hypoxia-inducible genes whose expression is linked to cancer progression[37]. This occurs in most tissues at normal oxygen levels by binding, ubiquitination and degradation of prolyl-hydroxylated α subunit of hypoxia-inducible transcription factor[38]. Under hypoxia, pVHL fails to recognize non-hydroxylated HIF-α what results in its stabilization, dimerization with a constitutive HIF-β subunit and transcriptional activation of the hypoxia-responsive targets[39,40]. Another mechanism that leads to accumulation of HIF-α and continuous activation of hypoxia-inducible genes involves biallelic deletions/mutations of VHL gene producing either non-functional or no pVHL. However, in contrast to ubiquitously operating hypoxic pathway, disabling mutations of VHL are confined to only several types of tumors, including renal cell carcinomas (RCC), hemangioblastomas, pheochromocytomas, endolymphatic-sac tumors, and islet-cell tumors of the pancreas[22]. The presence of such inactivating VHL mutations was demonstrated also in sporadic colorectal carcinomas[31], but the other insight into the same tumor type argues for the absence of the mutations[32]. Therefore their significance in these tissues remains unclear. In addition to functional aspects linking pVHL to control of hypoxic responses, which support the importance of pVHL status and tissue oxygenation, recent observations suggest that down-regulation of pVHL at the protein level may be also implicated in tumorigenesis[41]. However, this pVHL-related parameter has been so far assessed only in RCC and in the neoplastic lesions of the thyroid[42-44].
Carbonic anhydrases IX and XII have been first recognized as molecules down-regulated by pVHL in RCC cell lines expressing either the wild type or mutated VHL transgenes[6]. This finding explained overexpression of CA IX/CA XII in RCC. Further studies confirmed, at least for CA IX, that VHL mutations significantly correlate with CA9 expression in a series of RCC lines and that CA IX can identify early lesions in VHL kidneys[45,46]. Both CA IX and CA XII are subjected to pVHL control through HIF degradation also in normoxic tumor cells other than RCC and are induced in response to experimental as well as microenvironmental hypoxia[14,17]. Indeed, CA9 transcription is directly driven by HIF and a number of studies have shown the usefulness of CA IX protein as a marker of intratumoral hypoxia in a broad spectrum of tumors[14,15].
In the present work, we evaluated pVHL status and expression levels in a small cohort of patients with colorectal tumors in order to see whether there exists any noticeable link with the expression of CA IX and/or CA XII. An initial look at the immunohistochemical pattern of the three proteins confirmed previous observations that both CA IX and CA XII are consistently expressed in the basolateral membranes of the normal human colorectal epithelium, although being reciprocally distributed along the crypt-luminal surface axis[8,29,47]. CA IX is expressed only focally in the crypt enterocytes, whereas CA XII is predominantly found in the luminal part of the mucosa, i.e., the epithelial cuff region. In contrast, pVHL is quite evenly expressed throughout the mucosa from the deep to the surface regions and shows a cytoplasmic localization. Pathologic lesions exhibit higher variability mostly in the intensity of staining of each marker that can be explained by intratumoral heterogeneity, a general phenomenon resulting from ongoing tumorigenesis-related genetic and physiological alterations. Nevertheless, certain trends in the protein expression become apparent upon comparison of staining indices obtained in the normal tissues vs tumor counterparts even without discrimination between the deep and surface regions used in the previous studies. Increased CA IX expression shows a borderline association with the neoplastic lesions, but the increase in the present study was not as striking as shown before in the larger series of specimens[7]. In contrast, higher expression of CA XII is rather linked with normal tissues with a slight but significant decline in tumors. These associations are clearly evident also within individual differences found between the staining indices in tumors and corresponding normal specimens. While more than half of the patients display increase in CA IX, the majority shows decrease in CA XII protein levels. The same relationship is also true for CA9 and CA12 mRNAs. This divergent expression pattern is reminiscent of the situation in other tissues, particularly in kidney and breast, where CA IX expression is linked with carcinomas, while CA XII is frequently found both in normal tissues and tumors[16,26,28].
When considering entire groups of the pathologic lesions in comparison to normal tissues, pVHL expression appears essentially unchanged. However, separation of the data belonging to individual persons shows marked differences in the protein levels, with lowered values in several tumors in the absence of deletions/mutations as confirmed by the sequencing of VHL-specific RT-PCR products. Nevertheless, there is no obvious relationship between the levels of pVHL and either CA IX or CA XII. Of course, such a finding does not exclude the existence of the functional connection of pVHL with its targets in normal colorectal epithelium, which is uncoupled in tumors by hypoxia as explained above. This possibility has been explored by conducting semi-quantitative RT-PCR on mRNAs isolated from both normal and corresponding tumor tissue specimens. Such an approach has allowed for parallel amplification of CA9 and CA12-specific cDNAs using the same template. Simultaneously, VEGF mRNA expression has been assessed as an indicator of hypoxia, what was based on significant correlation between the accumulation of HIF-1α and induction of VEGF transcription in many tumor types including colorectal carcinomas[37]. In our study, the majority of the tumors with increased CA9 mRNA level also show higher level of VEGF mRNA supporting an involvement of hypoxia, but elevated levels of VEGF mRNA are equally prevalent in those pathologic lesions that display diminished CA9 expression. This naturally leads to lack of association between CA9 and VEGF and indicates participation of both common and different regulatory factors in control of these two genes. This is in accordance with their concordant but not fully overlapping expression in ovarian, head/neck, lung and bladder carcinomas[14,48,49]. Both genes are activated by microenvironmental hypoxia, but show differences upon reoxygenation, related particularly to a higher magnitude of transcriptional activation and prolonged persistence of CA IX protein[50]. They are also both induced by increased cell density[51-54]. Moreover, VEGF expression is induced by low glucose under normoxia, while CA IX responses to adverse microenvironmental stresses only in hypoxia[50,55].
Interestingly, a significant inverse relationship came out between the changes in the mRNA levels of CA12 and VEGF despite a relatively low number of examined specimens. One possible explanation is offered by a proposal made in breast tissues that factors related to differentiation dominate the regulation of CA XII[16]. In contrast, VEGF expression is preferentially linked to hypoxia and oncogenic pathways[54,56-58]. In agreement with these proposals, expression of CA XII is a marker of good prognosis in breast carcinomas and VEGF just as CA IX are markers of poor prognosis in different tumor types[16,18,59].
Altogether, neither VEGF up-regulation as the indicator of hypoxia nor VHL inactivating mutation seem to represent a simple missing link between pVHL and CA IX/CA XII in colorectal tumors. It is possible that such a direct link does not exist in tumors other than those associated with genetic inactivation of VHL tumor suppression. The definitive conclusion requires analysis of a considerably extended series of specimens. In the non-VHL tumors, other factors underlying overall tumor phenotype and intratumoral heterogeneity including oncogenic activation, stimulation by external growth factors and cytokines, pH and ion gradient etc., may account for the concurrent presence of high levels of pVHL and CA IX/CA XII in some tissues, and their contrasting expression pattern elsewhere. Thus, the relationship between these proteins cannot be identified merely by assessment of their expression levels and warrants parallel evaluation of other potential molecular players in this complex phenomenon.
ACKNOWLEDGMENTS
We wish to thank Dr. Berton Zbar (Laboratory of Immunobiology, Center for Cancer Research, National Cancer Institute at Frederick, MD) for the generous gift of pVHL-specific monoclonal antibodies.
Footnotes
Supported by the Grants From Sigrid Juselius Foundation, from Finnish Cultural Foundation and Finnish Dental Society, from the National Institutes of Health (DK40163, GM34182, and GM53405),from Slovak Grant Agency (2/2025), from the Slovak Government (Cancer Genomics SP 51/0280800), and from Bayer Corporation
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 2.Závada J, Závadová Z, Pastoreková S, Ciampor F, Pastorek J, Zelník V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–274. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 3.Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, Zat'ovicová M, Liao S, Portetelle D, Stanbridge EJ. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- 4.Opavský R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, Kettmann R, Pastorek J. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 5.Türeci O, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA. 1998;95:7608–7613. doi: 10.1073/pnas.95.13.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastoreková S, Pastorek J, Kairaluoma MI, Karttunen TJ. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivelä A, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156:577–584. doi: 10.1016/S0002-9440(10)64762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA. 2000;97:2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svastová E, Hulíková A, Rafajová M, Zat'ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–445. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors--Part 94. 1,3,4-thiadiazole-2-sulfonamidederivatives as antitumor agents? Eur J Med Chem. 2000;35:867–874. doi: 10.1016/s0223-5234(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 12.Supuran CT. Indisulam: an anticancer sulfonamide in clinical development. Expert Opin Investig Drugs. 2003;12:283–287. doi: 10.1517/13543784.12.2.283. [DOI] [PubMed] [Google Scholar]
- 13.Svastová E, Zilka N, Zat'ovicová M, Gibadulinová A, Ciampor F, Pastorek J, Pastoreková S. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res. 2003;290:332–345. doi: 10.1016/s0014-4827(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 14.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 15.Potter CP, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol. 2001;158:1011–1019. doi: 10.1016/S0002-9440(10)64048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson PH, Chia SK, Wykoff CC, Han C, Leek RD, Sly WS, Gatter KC, Ratcliffe P, Harris AL. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer. 2003;88:1065–1070. doi: 10.1038/sj.bjc.6600796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastorekova S, Pastorek J. Cancer-related carbonic anhydrase isozymes and their inhibition. In: Supuran CT, Scozzafava A, Conway J, eds. Carbonic Anhydrase, its Inhibitors and Activators. CRC Press Boca Raton London New York Washington DC; 2004. pp. 253–272. [Google Scholar]
- 20.Ohh M, Kaelin WG. The von Hippel-Lindau tumour suppressor protein: new perspectives. Mol Med Today. 1999;5:257–263. doi: 10.1016/s1357-4310(99)01481-1. [DOI] [PubMed] [Google Scholar]
- 21.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 22.Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 23.Karumanchi SA, Jiang L, Knebelmann B, Stuart-Tilley AK, Alper SL, Sukhatme VP. VHL tumor suppressor regulates Cl-/HCO3- exchange and Na+/H+ exchange activities in renal carcinoma cells. Physiol Genomics. 2001;5:119–128. doi: 10.1152/physiolgenomics.2001.5.3.119. [DOI] [PubMed] [Google Scholar]
- 24.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 25.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV. J Biol Chem. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- 26.Liao SY, Aurelio ON, Jan K, Zavada J, Stanbridge EJ. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–2831. [PubMed] [Google Scholar]
- 27.McKiernan JM, Buttyan R, Bander NH, Stifelman MD, Katz AE, Chen MW, Olsson CA, Sawczuk IS. Expression of the tumor-associated gene MN: a potential biomarker for human renal cell carcinoma. Cancer Res. 1997;57:2362–2365. [PubMed] [Google Scholar]
- 28.Parkkila S, Parkkila AK, Saarnio J, Kivelä J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Türeci O, Virtanen I, et al. Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem. 2000;48:1601–1608. doi: 10.1177/002215540004801203. [DOI] [PubMed] [Google Scholar]
- 29.Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, et al. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 30.Sakashita N, Takeya M, Kishida T, Stackhouse TM, Zbar B, Takahashi K. Expression of von Hippel-Lindau protein in normal and pathological human tissues. Histochem J. 1999;31:133–144. doi: 10.1023/a:1003554712386. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang Z, Emmert-Buck MR, Roth MJ, Gnarra J, Linehan WM, Liotta LA, Lubensky IA. von Hippel-Lindau disease gene deletion detected in microdissected sporadic human colon carcinoma specimens. Hum Pathol. 1996;27:152–156. doi: 10.1016/s0046-8177(96)90368-8. [DOI] [PubMed] [Google Scholar]
- 32.Miyakis S, Sourvinos G, Liloglou TL, Stathopoulos GP, Field JK, Spandidos DA. The Von Hippel-Lindau (VHL) tumor-suppressor gene is not mutated in sporadic human colon adenocarcinomas. Int J Cancer. 2000;88:503–505. [PubMed] [Google Scholar]
- 33.Pastoreková S, Závadová Z, Kostál M, Babusíková O, Závada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 34.Karhumaa P, Parkkila S, Türeci O, Waheed A, Grubb JH, Shah G, Parkkila A, Kaunisto K, Tapanainen J, Sly WS, et al. Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium. Mol Hum Reprod. 2000;6:68–74. doi: 10.1093/molehr/6.1.68. [DOI] [PubMed] [Google Scholar]
- 35.Nuorva K, Soini Y, Kamel D, Autio-Harmainen H, Risteli L, Risteli J, Vähäkangas K, Pääkkö P. Concurrent p53 expression in bronchial dysplasias and squamous cell lung carcinomas. Am J Pathol. 1993;142:725–732. [PMC free article] [PubMed] [Google Scholar]
- 36.Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, Ito M, Chayama K. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–181. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 37.Kim W, Kaelin WG. The von Hippel-Lindau tumor suppressor protein: new insights into oxygen sensing and cancer. Curr Opin Genet Dev. 2003;13:55–60. doi: 10.1016/s0959-437x(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 39.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 40.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 41.Shiao YH, Ramakrishna G, Anderson LM, Perantoni AO, Rice JM, Diwan BA. Down-regulation of von Hippel-Lindau protein in N-nitroso compound-induced rat non-clear cell renal tumors. Cancer Lett. 2002;179:33–38. doi: 10.1016/s0304-3835(01)00863-1. [DOI] [PubMed] [Google Scholar]
- 42.Shiao YH, Forsti A, Egevad L, Anderson LM, Lindblad P, Hemminki K. VHL down-regulation and differential localization as mechanisms in tumorigenesis. Kidney Int. 2003;64:1671–1674. doi: 10.1046/j.1523-1755.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi H, Miyamoto C, Furuichi Y, Yoshiike M, Nozawa S, Iwamoto T. VHL tumor suppressor gene: its mutation and protein level in renal cell carcinoma. Oncol Rep. 2003;10:1357–1361. [PubMed] [Google Scholar]
- 44.Hinze R, Boltze C, Meye A, Holzhausen HJ, Dralle H, Rath FW. Expression of the von Hippel-Lindau Tumor Suppressor Gene in Nonneoplastic and Neoplastic Lesions of the Thyroid. Endocr Pathol. 2000;11:145–155. doi: 10.1385/ep:11:2:145. [DOI] [PubMed] [Google Scholar]
- 45.Ashida S, Nishimori I, Tanimura M, Onishi S, Shuin T. Effects of von Hippel-Lindau gene mutation and methylation status on expression of transmembrane carbonic anhydrases in renal cell carcinoma. J Cancer Res Clin Oncol. 2002;128:561–568. doi: 10.1007/s00432-002-0374-x. [DOI] [PubMed] [Google Scholar]
- 46.Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/s1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 47.Pastoreková S, Parkkila S, Parkkila AK, Opavský R, Zelník V, Saarnio J, Pastorek J. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 48.Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–7998. [PubMed] [Google Scholar]
- 49.Turner KJ, Crew JP, Wykoff CC, Watson PH, Poulsom R, Pastorek J, Ratcliffe PJ, Cranston D, Harris AL. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs invasive bladder cancer. Br J Cancer. 2002;86:1276–1282. doi: 10.1038/sj.bjc.6600215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafajová M, Zatovicová M, Kettmann R, Pastorek J, Pastoreková S. Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol. 2004;24:995–1004. [PubMed] [Google Scholar]
- 51.Lieskovská J, Opavský R, Záciková L, Glasová M, Pastorek J, Pastoreková S. Study of in vitro conditions modulating expression of MN/CA IX protein in human cell lines derived from cervical carcinoma. Neoplasma. 1999;46:17–24. [PubMed] [Google Scholar]
- 52.Kaluz S, Kaluzová M, Chrastina A, Olive PL, Pastoreková S, Pastorek J, Lerman MI, Stanbridge EJ. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3'-kinase. Cancer Res. 2002;62:4469–4477. [PubMed] [Google Scholar]
- 53.Koura AN, Liu W, Kitadai Y, Singh RK, Radinsky R, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon carcinoma cells by cell density. Cancer Res. 1996;56:3891–3894. [PubMed] [Google Scholar]
- 54.Mukhopadhyay D, Tsiokas L, Sukhatme VP. High cell density induces vascular endothelial growth factor expression via protein tyrosine phosphorylation. Gene Expr. 1998;7:53–60. [PMC free article] [PubMed] [Google Scholar]
- 55.Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 57.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–498. [PubMed] [Google Scholar]
- 59.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol. 2001;2:667–673. doi: 10.1016/S1470-2045(01)00556-3. [DOI] [PubMed] [Google Scholar]


