Abstract
AIM: To analyze the influence factors and formation of extrahepatic collateral arteries (ECAs) in unresectable hepatocellular carcinoma (HCC) with or without chemoe-mbolization.
METHODS: Detailed histories of 35 patients with 39 ECAs of HCC and images including computerized tomography scan, digital subtraction angiography were reviewed carefully to identify ECAs of HCC, ECAs arising from, and anatomic location of tumors in liver. Tumor sizes were measured, and relations of ECAs with times of chemoemb-olization, tumor size, and the anatomic tumor location were analyzed. Complications were observed after chemoemb-olization through ECAs of HCC with different techniques.
RESULTS: Influence factors of formation of ECAs of HCC included the times of repeated chemoembolization, the location of tumors in liver, the tumor size and the types of chemoembolization. ECAs in HCC appeared after 3-4 times of chemoembolization (17.9%), but a higher frequency of ECAs occurred after 5-6 times of chemoem-bolization (56.4%). ECAs presented easily in peripheral areas (71.8%) of liver abutting to the anterior, posterior abdominal walls, the top right of diaphragm and right kidney. ECAs also occurred easily after complete obstruction of the trunk arteries supplying HCCs or the branches of proper hepatic arteries. Extrahepatic collaterals of HCC originated from right internal thoracic (mammary) artery (RITA, 5.1%), right intercostal artery (RICA, 7.7%), left gastric artery (LGA, 12.8%), right inferior phrenic artery (RIPA, 38.5%), omental artery (OTA, 2.6%), superior mesenteric artery (SMA, 23.1%), and right adrenal and renal capsule artery (RARCA, 10.3%), respectively. The complications after chemoembolization attributed to no super selective cathet-erization.
CONCLUSION: The formation of ECAs in unresectable HCC is obviously correlated with multiple chemoembolization, tumor size, types of chemoembolization, anatomic location of tumors. Extrahepatic collaterals in HCC are corresponding to the tumor locations in liver.
Keywords: ECAs, HCC
INTRODUCTION
Malignant liver tumors have a very poor prognosis. Primary hepatocellular carcinoma (HCC) is usually fatal, less than 5% of patients with HCC could survive five years after diagnosis. Although surgical resection is the standard treatment for HCC, disease-free survival of patients after curative resection of HCC remains poor due to the high recurrence of interhepatic tumor[1,2]. Most patients with HCC were at its advanced stage and would miss the optimal chance of operation. Therefore, the median survival time was only 4-6 mo for patients with unresectable tumors[3,4]. Systemic chemotherapy is relatively ineffective with a low response rate (20%), however, the mortality rate was up to 25%[5,6].
Transcatheter selective arterial treatment of liver tumors with chemotherapeutic and embolic agents (transarterial chemoembolization, TACE) for patients with unresectable HCC has been widely used as an effective therapeutic method, or as an alternative palliative treatment[7-11]. The results were superior to those of surgery in some series of patients with advanced HCC[12].
TACE cannot be considered as a radical treatment of HCC (even for diseases in the early stage), because primary malignant liver has an invasive growth pattern and can infiltrate into parenchyma through tumor capsules. Additionally, the efficacy of chemoembolization may descend after repeated TACE. One of the important factors affecting chemoembolization effect is the insufficient suppression of formation of extrahepatic collateral arteries(ECAs) in HCC. Malignant tumor tissues often acquire blood from ECAs, when normal supply from hepatic arteries is obstructed with TACE, occurring after multiple chemoembolization of unresectable HCC[15]. The influence factors and the formation of parasitic arteries in HCC were analyzed.
MATERIALS AND METHODS
Patients
Medical histories and records of 35 patients with unresectable HCCs, who developed extrahepatic collateral circulations after multiple TACE, were reviewed from July 1997 to Feb 2004. Of the 35 patients 33 were males (aged from between 31 to 71 years), two were females (aged between 45 and 65 years). Most patients with HCC had a relatively good hepatic function reserve without absolute contraindications for chemoembolization, hepatic function reserve of Child’s class was grade A in four cases, grade B in 29 cases, and grade C in two cases. The sizes of HCC varied from 3.5 to 13 cm in diameter, with an average diameter of 6.5 cm. Tumor sizes were estimated by computerized tomogram (CT) scan or digital substraction angiography. Tumor marker level of HCC (α-fetoprotein, AFP) was observed periodically pro-and post-chemoembolization with double radioimmunoele-ctrophoresis.
Means of identifying ECAs
Additional angiographies were performed besides regular celiac arteriography and superior mesenteric arteriography to identify ECAs, if the signals suggested formation of extrahepatic collaterals in HCC located in peripheral regions of liver abutting to anterior or posterior thoracic wall, right inferior diaphragm, and falciform ligament on CT scan, or HCCs showed defects of tumor staining on arterial angiogram. Additional angiographies included super selective arteriography of LGA, RITA, low RICA, RIPA, and sometimes right renal artery.
Criteria of tumor anatomic location
The criteria of tumor anatomic location were assessed by CT scan. Peripheral HCC means that tumors were adjacent to bare area and subcapsules in liver, whereas, central HCC means tumor located in the center of liver, being away from subcapsules or bare area on CT scan.
Administration patterns of chemoembolization
Most patients had undergone repeated chemoembolization before HCC formed extrahepatic collaterals in liver. The chemoembolization were typically performed in three steps as the following:
Firstly, regional chemotherapeutic perfusion to HCC was done with a combination of anticancer agents: the chemoth-erapeutic drugs 5-Floxuridine (5-FU) 750-1000 mg and Cisplatin (DDP) 60-80 mg were dissolved in 50-100 mL normal saline, respectively, then the solutions were separately perfused slowly into hepatic arteries and parenchyma of tumors within 30 min through catheters.
Secondly, chemoembolization of HCC was followed by the emulsion of iodized oil mixed with chemotherapeutic agent (Epirubicin, 40-60 mg). Iodized oil:Epirubicin: contrast medium were 5 mL:8 mg:1 mL, and a single dose of emulsified mixture ranged from 6 to 15 mL in chemoembolization, which depended on volume, numbers and rich stain of tumors after angiogram.
Finally, blood supply HCC were occluded in different ways: One was a selective occlusion of right or left hepatic arteries with absorbable gelatin sponge articles according to tumors in left or right lobe, if arterial portography showed no tumor thrombi blockage of main port vein; another was an occlusion artery of HCC with gelfoam powders of diameter 50-100 μm.
Chemoembolization of HCC had been almost finished with 5 F (French size) catheters before ECAs were found. However, if ECAs were found after multiple TACE, chemoemolization of HCC was mainly completed with transmicrocatheter via ECAs.
RESULTS
Level of AFP in seven cases persistently increased after multiple chemoembolization before ECAs were found in the follow-up period, which rose from 620-1250 μg/L. It usually implied that ECAs in HCC had formed. Then the formation of ECAs was frequently proved by additionally super-selective angiographies, besides angiograms in celiac arteries. Many factors could result in the formation of ECAs, which involved times of chemoembolization, tumor size, anatomic location of tumors in liver, and initially patterns of chemoembolization, etc.
Of all the 35 patients, 31 had accepted repeated chemoembolization of HCC before 37 ECAs (94.9%) among them were found by arteriogram; only three (7.7%) ECAs initially existed in HCC, which were diagnosed by enhanced CT scans before they underwent first angiogram and chemoembolization (Table 1). The data of Table 1 demonstrate that HCCs more easily formed extrahepatic collaterals after repeated treatments with TACE, especially after four-time chemoembolization than that before first chemoembolization of HCC and after few times of TACE.
Table 1.
Incidence of formation of ECAs occurred in different sizes of tumors and different times of TACE.
|
Tumor size (cm) |
Times of chemoembolization |
|||||||
| ≤5 | ≤10 | 10< | 0 | 1-2 | 3-4 | 5-6 | 7- | |
| Incidence of EHCA (%) | 17.9 (7/39) | 76.9 (30/39) | 5.1 ( 2/39) | 7.7 (3/39) | 5.13 (2/39) | 17.9 (7/39) | 56.4 (22/39) | 12.8 (5/39) |
After repeated chemoembolization, most of the ECAs in HCC, 76.9% (30/39), formed among the tumors whose sizes ranged from 5 to 10 cm (5<~≤10 cm) in diameter, 17.9% and 5.1% (2/39) among those sizes ≤5 cm and >10 cm, respectively. It suggested that tumor size was one of the crucial factors forming ECAs in HCC after multiple chemoembolization, as well.
Thirty-nine ECAs of HCC in 35 patients originated from RITA, RICA, LGA, RIPA, OTA, SMA, and RARCA, respectively (Table 2). Tumors that formed ECAs in peripheral and central areas in liver was separately 71.8% (28/39) and 28.2% (11/39). The formation of ECAs closely related to the location of HCC in edge liver: tumors adjacent to anterior abdominal wall usually acquired collateral supply from RITA, 5.1% (2/39); OTA, 2.6% (1/39); partial SMA, 15.2% (6/39); RIPA, 12.8% (5/39). HCC abutting the right lateral or posterior abdominal wall frequently obtained extrahepatic collateral supply from RICA, 7.7% (3/39); partial RIPA, 6.7% (2/39). RIPA were main sources of ECAs in HCC, when tumors located in the top of right diaphragm, 20.5% (8/39). A few tumors in liver adjacent to right adrenal gland and kidney were more often fed by RARCA, 10.3% (4/39). All those mean that the anatomic location of tumors in liver was also an important factor for formation of ECAs in HCC. It was easier for HCCs to form ECAs in the periphery than in the central part of the liver after multiple chemoembolization.
Table 2.
Incidence of ECAs of tumors occurred in different anatomic areas in liver and from different origin.
|
Location of tumor |
Origination of ECAs |
||||||||
| Peripheral | Central | RITA | RICA | LGA | RIPA | SMA | OTA | R ARCA | |
| Incidence of EHCA (%) | 71.8 | 28.2 | 5.1 | 7.7 | 12.8 | 38.5 | 23.1 | 2.6 | 10.3 |
| (28/39) | (11/39) | (2/39) | (3/39) | (5/39) | (15/39) | (9/39) | (1/39) | (4/39) | |
Patterns of chemoembolization influenced the formation of ECAs in HCC, as well. 30.8% (12/39) ECAs in HCC appeared between the first and the fourth chemoembolization, which occurred relatively early during the whole course of treatment after occlusion of the proximal arteries supplying tumors and proper hepatic arteries with gelatin sponge articles through 5 F-catheter. Whereas, chemoembolization was performed with emulsion of iodized oil mixed with anticancer drugs and then followed by gelfoam powder blocking blood feeding of tumors and distal branches of hepatic arteries trans-microcatheter, ECAs in HCC frequently came into being latterly (after fifth chemoembolization), but the incidence of ECAs of HCC was relatively higher, 69.2% (27/39).
Only 11 ECAs in tumors were successfully embolized with 5 F catheter, which were 5 SMAs, 4 RIPAs and 2 LGAs, respectively; 28 ECAs were performed with 3 F microcatheter. Complications appeared in four patients, two had persistent hiccups for a week after chemoembolization of RIPA with 5 F-catheter, one with portal venous tumor thrombi had aggressive hepatic failure and hepatic encephalopathy in 3 d after occlusion SMAs with 5 F catheter, one had erythema of skin after embolization of RITA with microcatheter, in which the tip of microcatheter could not reach the distal end of extrahepatic collateral artery.
DISCUSSION
TACE has been considered as an effective treatment for unresectable HCC or as a compensation of postoperatively recurrent HCC nowadays[14,15], sometimes, even as an alternative for resectable HCC[16].
Volume of HCC could not be reduced, moreover, it became larger and hepatic function reserve got worse than ever before, and the serum level of tumor marker for HCC (AFP) continuously increased after repeated chemoembolization in some patients. All those suggest that HCC might have formed the ECAs in liver after multiple chemoembolization, which was usually neglected, and the above changes could not be considered as signals of ECAs formation in HCC. In our documents, persistent increase of serum AFP level in some patients after repeated chemoembolization had not been connected with the formation of ECAs in HCC until additional angiograms were performed in the following procedures. ECAs in HCC might have existed and could have been neglected in past treatments, they also probably formed in interphases of chemoembolization. Therefore, we can regard the changes as messages of ECAs possibly forming in HCC after TACE.
As the incidence of ECAs in HCC concerned, Li et al[7,17], reported that extrahepatic blood supply tumors could be found in 43.1% patients with unresectable HCC; Okazaki et al[18,19], showed that one or more focuses of interhepatic recurrence of HCC after hepatectomy in 38.2% patients (26/68) were fed not only by hepatic arteries but also by ECAs; another article reported that ECAs forming in HCCs only reached 11.4% (19/167)[20]. Although it was very different among reported articles that the incidence of ECAs forming in HCC occurred after chemoembolization, it was still high and ECAs had influenced the efficacy of chemoembolization of unresectable HCC. So it needs us to pay more attention to find out and process ECAs in HCC after multiple chemoembolization.
Finding out ECAs of HCC becomes the crucial factor to consolidate and further enhance the effect of interventional therapy of HCC after obstruction of main blood supply to the tumors. Therefore, it is necessary for performers to fully understand variant anatomy of normally hepatic arteries, especially to know where ECAs in unresectable HCC potentially originate from after multiple chemoembolization[21]. Our data demonstrate that ECAs feeding HCC mainly come from the right inferior phrenic arteries and the superior mesenteric arteries, both accounted for more than half of the 39 ECAs. The results also showed that ECAs in HCC could originate from the left gastric arteries and the right adrenal and renal capsular arteries, which should not be neglected. Some extrahepatic collaterals in HCC were relatively uncommon such as arising from the right internal thoracic arteries, right intercostal arteries and omental arteries. All styles and the incidence of formation of ECAs in HCC in this article were almost consistent with the data reported by Chung[22]. ECAs in HCC, however, could be rarely found by routine celiac arterial angiogram[23-25], particularly, the origination of ECAs in HCC from right renal arteries was occasionally displayed[26,27]. The ECA in one case of our data was found originating from the right renal artery by chance, because the tip of the catheter rebounded back to abdominal aorta from celiac artery while injecting the contrast medium (Figure 1). Therefore, it is necessary for us to consider superior mesenteric arterial, right inferior phrenic arterial, left gastro arterial and right renal arterial angiograms as regular processes in the course of next treatment after multiple chemoembolization. Selective right internal thoracic arteries and right intercostal arteries angiographies may be concerned when there exist potential factors of ECAs form in HCC, such as signs of filling defects in tumors during capillary phase after injection of contrast median, insufficient ethiodized oil retention, and tumor located in the periphery of liver. Sometimes enhanced CT scan or serum level of tumor marker (AFP) during follow-up may also be regarded as clues to finding those potential extrahepatic collaterals. Enhanced CT scan often showed that ECAs in HCC appeared around the liver under capsula fibrosa (Figure 2).
Figure 1.
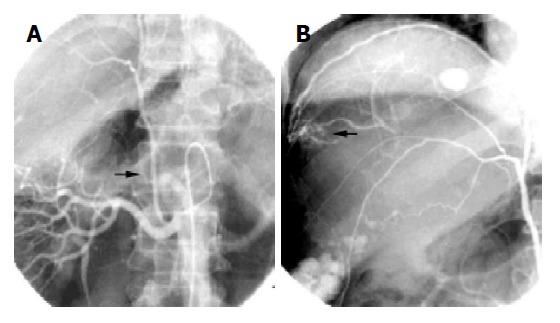
A and B: the same case. A: ECA of HCC originated from right renal artery (arrow); B: Tumor was fed by hypertrophy ECA from right renal artery (arrow).
Figure 2.
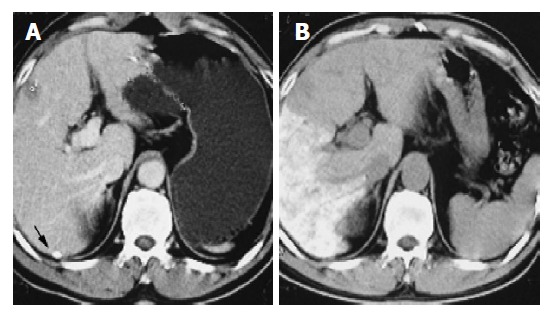
A: Pro-chemoembolization, enhanced CT scan showed ECA of HCC under subcapsule of liver (arrow); B: Ethiodized oil stayed at tumor area via the ECA of HCC after chemoembolization.
Anatomic location of HCC in liver determines the formation and styles of ECAs in tumors. Some advanced or superficial tumors of liver often get arterial blood from adjacent organs after the multiple procedures[17,23]. Our findings demonstrate, as well, that HCCs located in the peripheral areas (71.8%) are easier to form ECAs than that located in the center regions (28.2%) in liver. Each artery supplying normal tissues or organs abutting superficial HCC in liver might become a latent ECA of HCC, when main blood supply to tumors from left or right hepatic arteries is completely occluded after chemoembolization. Thus, ECAs in HCC variedly originated from RITA, RICA, RIPA, LGA, SMA, OTA and RARCA, which was the anatomic location of tumors in liver that decided what kind of styles of ECAs emerged post-chemoembolization[20,22].
Our findings showed that the incidence of ECAs in HCC began to increase after the third or fourth chemoembolization (17.9%), and reached the highest after the fifth or the sixth chemoembolization. Significant differences presented at different times of chemoembolization. This suggests that there might be a close correlation between ECAs in HCC and times of chemoembolization. The more the times of chemoembolization, the higher the formation of ECAs in HCC. However, the rate of ECAs in HCC accounting in the whole group reversely descended (12.8%) after the seventh chemoembolization. The reason why the formation of ECAs in HCC decreased after too many times of chemoembolization might be caused by the reduction of the survivals with advanced HCC after repeated interventional therapy and decrease of the numbers of patients who could not bear further chemoembolization due to poor functional reserve after the seventh TACE.
There a phenomenon appeared when we analyzed the relationship between ECAs in HCC and tumor size. The ECAs forming in HCC was positively proportional to tumor size which was lesser than 10 cm in diameter, and was negatively proportional to the size which was larger than 10 cm in diameter. The reason used to illustrate how many times chemoembolization increased and the formation of ECAs decreased in HCC after the seventh TACE also could be used to explain why the negative proportion of ECAs appeared when tumor size was larger than 10 cm in diameter.
Generally speaking, we could conclude that the rate of ECAs forming in HCC was also closely correlated to tumor size as well as times of multiple chemoembolization.
If complications were reduced as quickly as depended on occlusion of ECAs in HCC without blocking the branches supplying normal tissues and organs when we wanted to make further treatment of ECAs after TACE[28]. After multiple chemoembolization, most superficial tumors acquired the extrahepatic arterial blood which supplied adjacent organs and tissues. Micro-catheters must be used in super selective chemoembolization if ECAs originate from right kidney, inferior phrenic, internal thoracic, omental, and intercostal arteries. Those branches are usual tortuous and hypertrophy is not enough to pass common size catheters (5-6 F) to distal of ECAs (Figures 1 and 3). So, complications often occur after the chemoembolization without avoiding the blood supplying normal tissues[29-33]. Obstinate hiccups, progressive loss of hepatic function reserve, and even hepatic encepha-lopathy appeared in our findings, which was attributable to chemoembolization with 5-6 F catheter resulting in occlusion of blood feeding normal tissues. The erythema and the necrosis of skin after chemoembolization were not uncommon even with the tip of microcatheter placed in distal intercostal artery[13]. Thus, it is important to recognize that not all such vessels can be safely treated without the risk of injury of normal tissues, or even more important organs. Indications recomm-ended for embolization of these extrahepatic collaterals is the “back of the room” rule, which means that tumor blood supply from these vessels is obvious and super selective catheterization[13,34]. However, it is worthwhile for patients with advanced HCC to take the risk if we carefully occlude ECAs of tumors with a much thinner tip of microcatheter such as neurointerventional microcatheter (Figure 4)[35-37].
Figure 3.
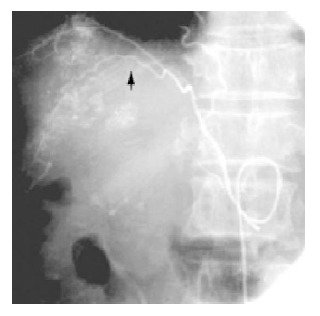
ECA of HCC originated from right inferior phrenic artery (arrow).
Figure 4.
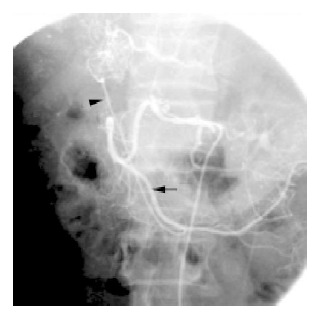
ECA (arrowhead)of HCC originated from omental artery (arrow).
In conclusion, our observations indicate that the styles and the formation of ECAs in HCC are closely related to multiple chemoembolization, superficially anatomic location of tumors, and tumor size. Extrahepatic collateral supply HCCs is necessary to be treated, but they should be carefully embolized by superselective catheterization with microcatheter.
ACKNOWLEDGMENTS
The authors are grateful to professors Lin-Sun Li, First People’s Hospital Affiliated to Nan Jin Medical University, and Neng-Shu He, General Hospital of Tianjin Affiliated to Tianjin Medical University, for data sources.
Footnotes
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Sturm JW, Keese M. Multimodal treatment of hepatocellular carcinoma (HCC) Onkologie. 2004;27:294–303. doi: 10.1159/000077982. [DOI] [PubMed] [Google Scholar]
- 2.Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 3.Chen XM, Luo PF, Lin HH, Zhou ZJ, Shao PJ, Fu L, Li WK. Long-term result of combination of transcatheter arterial chemoembolization and percutaneous ethanol injection for treatment of hepatocellular carcinoma. AiZheng. 2004;23:829–832. [PubMed] [Google Scholar]
- 4.Jang JW, Park YM, Bae SH, Choi JY, Yoon SK, Chang UI, Nam SW, Kim BS. Therapeutic efficacy of multimodal combination therapy using transcatheter arterial infusion of epirubicin and cisplatin, systemic infusion of 5-fluorouracil, and additional percutaneous ethanol injection for unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:415–420. doi: 10.1007/s00280-004-0829-7. [DOI] [PubMed] [Google Scholar]
- 5.Testa R, Testa E, Giannini E, Botta F, Malfatti F, Chiarbonello B, Fumagalli A, Polegato S, Podesta E, Romagnoli P, et al. Trans-catheter arterial chemoembolisation for hepatocellular carcinoma in patients with viral cirrhosis: role of combined staging systems, Cancer Liver Italian Program (CLIP) and Model for End-stage Liver Disease (MELD), in predicting outcome after treatment. Aliment Pharmacol Ther. 2003;17:1563–1569. doi: 10.1046/j.1365-2036.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 6.Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, Amesur N, Zajko A. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:1042–1050. doi: 10.1002/cncr.11111. [DOI] [PubMed] [Google Scholar]
- 7.Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003;12:105–126. doi: 10.1016/s1055-3207(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 8.Trinchet JC, Ganne-Carrie N, Beaugrand M. Review article: intra-arterial treatments in patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17 Suppl 2:111–118. doi: 10.1046/j.1365-2036.17.s2.19.x. [DOI] [PubMed] [Google Scholar]
- 9.Xiao E, Li D, Shen S, Zhou S, Tan L, Wang Y, Luo J, Wu Y, Tan C, Liu H, et al. Effect of preoperative transcatheter arterial chemoembolization on apoptosis of hepatocellular carcinoma cells. Chin Med J (Engl) 2003;116:203–207. [PubMed] [Google Scholar]
- 10.O'Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, Chung JW, Park JH, Kim CY. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol. 2002;20:4459–4465. doi: 10.1200/JCO.2002.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi H, Ushiyama T, Ishimura K, Izuishi K, Karasawa Y, Masaki T, Watanabe S, Kuriyama S, Maeta H. Significance of reduction surgery in multidisciplinary treatment of advanced hepatocellular carcinoma with multiple intrahepatic lesions. J Surg Oncol. 2003;82:98–103. doi: 10.1002/jso.10203. [DOI] [PubMed] [Google Scholar]
- 13.Park SI, Lee DY, Won JY, Lee JT. Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J Vasc Interv Radiol. 2003;14:461–468. doi: 10.1097/01.rvi.0000064856.87207.1e. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki Y, Imaoka S, Nakano H, Tamura S, Aono Y, Furukawa H, Ishikawa O, Kabuto T, Hiratsuka M, Kameyama M, et al. Hepatic resection with "wrapping therapy" for advanced hepatocellular carcinoma uncontrolled by arterial embolization. Gan To Kagaku Ryoho. 1996;23:1588–1591. [PubMed] [Google Scholar]
- 15.Kang IK, Kim SW, Hahn SH, Cho SC, Gham CW, Lee DH. A comparison of patients with hepatocellular carcinoma between a short-term (less than 6 months) survival group and a long-term (over 24 months) survival group after treatment with transcatheter arterial chemoembolization. Taehan Kan Hakhoe Chi. 2002;8:189–200. [PubMed] [Google Scholar]
- 16.Yuen MF, Chan AO, Wong BC, Hui CK, Ooi GC, Tso WK, Yuan HJ, Wong DK, Lai CL. Transarterial chemoembolization for inoperable, early stage hepatocellular carcinoma in patients with Child-Pugh grade A and B: results of a comparative study in 96 Chinese patients. Am J Gastroenterol. 2003;98:1181–1185. doi: 10.1111/j.1572-0241.2003.07404.x. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Guo Y, Tian G, Shi Z, Liu D, Zeng H, Jiang W, Li H, Zhou C. Extrahepatic arterial blood supply of hepatocellular carcinoma and interventional treatment. Zhonghua ZhongLiu ZaZhi. 2002;24:163–166. [PubMed] [Google Scholar]
- 18.Puleo S, Mauro L, Gagliano G, Lombardo R, Li Destri G, Petrillo G, Di Carlo I. Liver damage after transarterial chemoembolization without embolizing agent in unresectable hepatocellular carcinoma. Tumori. 2003;89:285–287. doi: 10.1177/030089160308900310. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki M, Yamasaki S, Ono H, Higashihara H, Koganemaru F, Kimura S, Kuroda Y, Sato S, Ryu K, Ohtsubo T. Chemoembolotherapy for recurrent hepatocellular carcinoma in the residual liver after hepatectomy. Hepatogastroenterology. 1993;40:320–323. [PubMed] [Google Scholar]
- 20.Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T. Angiographic classification of hepatic arterial collaterals. Radiology. 1982;144:485–494. doi: 10.1148/radiology.144.3.6285413. [DOI] [PubMed] [Google Scholar]
- 21.Shibata T, Kojima N, Tabuchi T, Itoh K, Konishi J. Transcatheter arterial chemoembolization through collateral arteries for hepatocellular carcinoma after arterial occlusion. Radiat Med. 1998;16:251–256. [PubMed] [Google Scholar]
- 22.Chung JW, Park JH. Extrahepatic collaterals in hepatocellular carcinoma. Man Chung Han, Jae Hyung Park, eds. Interventional therapy. Seoul Korea. 1999:133–145. [Google Scholar]
- 23.Eurvilaichit C. Outcome of transcatheter oily chemoembolization in patients with hepatocellular carcinoma. Hepatogastroenterology. 2004;51:20–24. [PubMed] [Google Scholar]
- 24.Miyayama S, Matsui O, Nishida H, Yamamori S, Minami T, Shinmura R, Kozaka K, Notsumata K, Toya D, Tanaka N, et al. Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol. 2003;14:1155–1161. doi: 10.1097/01.rvi.0000086534.86489.10. [DOI] [PubMed] [Google Scholar]
- 25.Gates J, Hartnell GG, Stuart KE, Clouse ME. Chemoembolization of hepatic neoplasms: safety, complications, and when to worry. Radiographics. 1999;19:399–414. doi: 10.1148/radiographics.19.2.g99mr08399. [DOI] [PubMed] [Google Scholar]
- 26.Kim DE, Yoon HK, Ko GY, Kwon JS, Song HY, Sung KB. Hepatic falciform artery: is prophylactic embolization needed before short-term hepatic arterial chemoinfusion? AJR Am J Roentgenol. 1999;172:1597–1599. doi: 10.2214/ajr.172.6.10350296. [DOI] [PubMed] [Google Scholar]
- 27.Okino Y, Kiyosue H, Matsumoto S, Takaji R, Yamada Y, Mori H. Hepatocellular carcinoma: prediction of blood supply from right inferior phrenic artery by multiphasic CT. J Comput Assist Tomogr. 2003;27:341–346. doi: 10.1097/00004728-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Miyayama S, Matsui O, Taki K, Minami T, Ito C, Shinmura R, Takamatsu S, Kobayashi M, Notsumata K, Toya D, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma fed by the reconstructed inferior phrenic artery: anatomical and technical analysis. J Vasc Interv Radiol. 2004;15:815–823. doi: 10.1097/01.RVI.0000136986.34890.D7. [DOI] [PubMed] [Google Scholar]
- 29.Seki S, Kitada T, Sakaguchi H, Nakatani K, Kamino T, Nakamura K, Yamada R. Cardiac tamponade caused by spontaneous rupture of mediastinal lymph node metastasis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16:702–704. doi: 10.1046/j.1440-1746.2001.02375.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Chon CY, Ahn SH, Moon BS, Kim JH, Paik YH, Han KH, Lee JT, Lee DY, Moon YM. An ischemic skin lesion after chemoembolization of the right internal mammary artery in a patient with hepatocellular carcinoma. Yonsei Med J. 2001;42:137–141. doi: 10.3349/ymj.2001.42.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Nakai M, Sato M, Kawai N, Minamiguchi H, Masuda M, Tanihata H, Takeuchi T, Terada M, Kishi K. Hepatocellular carcinoma: involvement of the internal mammary artery. Radiology. 2001;219:147–152. doi: 10.1148/radiology.219.1.r01mr28147. [DOI] [PubMed] [Google Scholar]
- 32.Liu HJ, Chen TS, Lee RC, Ho DM, Lin JT, Chu LS, Chang FY. Abdominal wall necrosis following transcatheter arterial chemoembolization for hepatocellular carcinoma. Zhonghua YiXue ZaZhi (Taipei) 2000;63:838–843. [PubMed] [Google Scholar]
- 33.Memis A, Oran I, Calli C, Yuzer Y, Mentes A. Transcatheter arterial embolization of internal mammary artery in a hepatocellular carcinoma with abdominal wall invasion. Eur J Radiol. 1998;28:160–163. doi: 10.1016/s0720-048x(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 34.Kanetsuki I, Hori A, Ohshiro K, Nishi H, Yasutani T, Sueyoshi T, Tanaka H. Left lobe recurrent hepatocellular carcinoma treated with lipiodol-TAE via the left internal mammary artery. Cardiovasc Intervent Radiol. 1997;20:387–389. doi: 10.1007/s002709900174. [DOI] [PubMed] [Google Scholar]
- 35.Eurvilaichit C, Chuapetcharasopon C. Hepatic arterial collaterals after transcatheter oily chemoembolization of hepatocellular carcinoma. J Med Assoc Thai. 2001;84:75–84. [PubMed] [Google Scholar]
- 36.Sasaki Y, Imaoka S, Shibata T, Ishikawa O, Iwanaga T, Kasugai H, Fujita M. Decollateralization with silicone rubber sheeting for advanced hepatocellular carcinoma: a preliminary report. Surgery. 1990;108:840–846. [PubMed] [Google Scholar]
- 37.Won JY, Lee DY, Lee JT, Park SI, Kim MJ, Yoo HS, Suh SH, Park SJ. Supplemental transcatheter arterial chemoembolization through a collateral omental artery: treatment for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2003;26:136–140. doi: 10.1007/s00270-002-2629-y. [DOI] [PubMed] [Google Scholar]


