Abstract
AIM: To investigate the therapeutic effects of emodin in combination with baicalein on severe acute pancreatitis (SAP) rats and to explore the mechanism of SAP.
METHODS: A total of 112 SAP rats induced by retrograde injection of 5% sodium taurocholate into the biliary-pancreatic duct, randomly assigned to a untreated group and three treated groups emodin group, combined emodin and baicalein group, and sandostatin group. Meanwhile, another 28 other rats were selected as sham operation (SO) group. There were 28 rats in each group, 8 rats were in 3 and 6 h groups respectively, and 12 rats in 12 h group. At each time-points, survival rates, ascites volumes, pathological lesion scores of pancreas tissues, serum amylase, tumor necrosis factor-α and IL-6 levels were determined as the indexes of therapeutic effects.
RESULTS: The survival rate at 12 h was significantly higher in three treated groups than in untreated group. The ascites volume at 12 h was remarkably less in combined and sandostatin groups than in emodin group, but there was no difference between combined group and sandostatin group (P>0.05). Serum amylase levels at all time-points were significantly lower in three treated groups than in untreated group. However, they had no difference among treated groups (P>0.05). Serum TNF-α were lower in three treated groups than in untreated group at all time points. Among the three treated groups, at 6 h, the TNF-α levels of combination and sandostatin groups were lower than those of emodin group. These was no difference between combined and sandostantin. Serum IL-6 concentration at 3 h were lower in combined and sandostatin groups than in untreated group, but at 6 and 12 h they were lower in all treated groups than in untreated group and the combined and sandostatin groups and in emodin group, no difference was found between combined and sandostatin groups at all time-points (P>0.05). The pathological scores of pancreas at all time points were significantly lower in three treated groups than in the untreated group, and at 6, 12 h, the scores of combined and sandostatin groups were lower than in emodin group. There was no difference between combined and sandostatin groups (P>0.05).
CONCLUSION: Combination of emodin with baicalein has significant therapeutic effects on SAP rats.
Keywords: Emodin, Severe acute pancreatitis
INTRODUCTION
Severe acute pancreatitis (SAP) has high incidence of complications and high mortality. For many years, treating SAP has been difficult. Up to now, some drugs for treating SAP have been used in the clinic, especially sandostatin, an analog to somatostatin has rather excellent effects on inhibiting pancreatic excretion and preventing complications. However, it is too expensive, especially in remote poor areas. So, it is necessary to find some cheap and high effective drugs. Emodin is a main active monomer of rhubarb, which is widely used in traditional Chinese herb as laxative, is a derivative of anthraquinone (3-methyl-1,6,8-trihydroxyanthraquinone). Baicalein is a main active monomer in herb Scutellaria baicalensis (baikal skullcap root), and belongs to glucuronid category[1,2]. A lot of studies have confirmed[3-5] that intravenous injection of emodin has some curative effects on SAP rats. Our previous research on the treatment of SAP rat using intravenous injection of baicalein showed that baicalein could attenuate pancreatic pathological lesions and reduce SAP mortality. From Chinese traditional medicine view, the effect of complex-prescriptions is better than that of simple ones. For this reason, we investigated the curative effect of combined emodin with baicalein on SAP rats and explored its mechanism.
MATERIALS AND METHODS
Main drugs and reagents
Sodium taurocholate purchased from Sigma Company was diluted to 5% solution before application. Emodin from Beijing Biological Products Clinical Laboratory, baicalein extracted from Baikal skullcap root by Pharmaceutical College of Xi’an Jiaotong University, China (purity>98%, HPLC), were prepared to 0.25% and 1% injection solution, respectively. The national invention patents have applied for the formula of Emodin injection solution and Baicalein injection solution. The patent numbers are 200410016809.X and 200410016810.2, respectively. Sandostatin was purchased from Novartis Pharma Company, Switzerland, and the test kit of amylase was obtained from Nanjing Jiancheng Bioengineering Institute. The test kits of TNF-α and IL-6 were purchased from Shanghai Shensu Biotech Company, China.
Experimental rats
Healthy male sanitary SD rats weighing 250-335 g were supplied by Animal Experimental Center of Shanxi Research Institute of Chinese Traditional Medicine, Shanxi Province, China. A total of 112 SAP rats induced by retrograde injection 5% sodium taurocholate into biliary-pancreatic duct, were randomly divided into an untreated group and three treated groups: emodin group combined emodin with baicalein group, and sandostatin group. Each group contained 28 SAP rats. Meanwhile another 28 rats were selected to perform exploratory laparotomy as sham operation (SO) group. The 28 rats in each group were further divided into three subgroups; postoperative 3 h (8 rats), 6 h (8 rats) and 12 h (12 rats) groups. At the above-correspondent time-points these rats were killed by cervical decapitation. Blood and pancreas tissue samples of the killed rats were studied.
Operative procedures and treatment
Before operation, the rats were fasted for 12 h, but allowed to drink water freely. They were anesthetized by 20% urethane intraperitoneal injection (0.5 mL/100 g) as routine. A thigh vein cannulation was set up, and through it NS (sodium chloride) solution was successively supplied at 1 mL/100 g/h speed. After abdomen was opened, according to the calculation of 4 mg/100 g, 5% sodium taurocholate was retrograde injected into the biliary-pancreatic duct at 10 mg/min speed to induce the SAP model. Meanwhile, exploratory laparatomy was performed on the rats in SO group and their pancreas was gently drawn, then the abdomen was closed. After that, emodin (0.25 mg/100 g/6 h), baicalein (2 mg/100 g/6 h) and sandostatin (0.2 μg/100 g/6 h) were transfused into the rats of each correspondent group.
Observatory indexes and measurement methods
To observe the survival of rats at each time-point, the fluid from the peritoneal cavity was taken using absorbent cotton, weighed before and after operation, and the difference of weights (g) was converted to mL. The tissue samples were fixed in 40 g/L formaldehyde, then sliced and stained with HE. Under microscopy, the pathological lesion scores were evaluated by the double blind method, the assay standard of the scores was acted in methods of Spomam[6] (Table 1). The level of serum amylase was determined by iodine-amylum chromatometry. The serum TNF-α and IL-6 levels were assayed by ABC-ELISA.
Table 1.
The standard of pathological score of pancreas.
| Histological changes | Classification | Score |
| Edema | Lobar diaphragm widen | 1 |
| Lobar + interlobular septae widen | 2 | |
| Lobar + lobulous + interacinar septae widen | 3 | |
| Infiltration of inflammatory cell | 1-10 leukocyte / HPF | 1 |
| 11-20 leukocyte / HPF | 2 | |
| >20 leukocyte / HPF | 3 | |
| Fat necrosis | <2 necrotic cells / per lobule | 3 |
| 3-5 necrotic cells / per lobule | 5 | |
| >5 necrotic cells / per lobule | 7 | |
| Parenchymatous necrosis | Necrotic area <5% total area | 3 |
| Necrotic area accout 5-20% total area | 5 | |
| Necrotic area>20% total area | 7 | |
| Hemorrhagic focus | <2 focus / per lobule | 3 |
| 2-4 focus / per lobule | 5 | |
| >4 focus / per lobule | 7 |
Note: HPF = 400× microscopy.
Statistical analysis
The results were presented as mean±SD. The χ2 test was used to compare the difference of survival rates. The difference among various groups was assessed by variance analysis, and P<0.05 was considered statistically significant. Computations were performed with SPSS software.
RESULTS
Survival rate
At 3 and 6 h, the rats in all groups were alive. At 12 h, the survival rates of the rats in SO group and three treated groups were 100%, and 50% in untreated group. So the survival rate was significantly higher in treated groups than in untreated group (P<0.05) (Table 2).
Table 2.
Survival rate of each group.
| Groups | 3 h (n = 8) | 6 h (n = 8) | 12 h (n = 12) |
| SO | 8 | 8 | 12a |
| Untreated | 8 | 8 | 6 |
| Emodin | 8 | 8 | 12a |
| Combined | 8 | 8 | 12a |
| Sandostatin | 8 | 8 | 12a |
P<0.05, vs the untreated group.
Ascites volume
The ascites volume at 3 h was not different between untreated group and treated groups (P>0.05). At 6 h, the ascites volume in sandostatin group was significantly less than that in untreated group (P<0.05). At 12 h, the volume was significantly less in three treated groups than in emodin group (P<0.01), and it was even more significant in the combined group and sandostatin groups than in untreated group (P<0.01), but no difference was found between combined and sandostatin groups (P<0.05). However, the ascites volume in untreated and treated groups was more than that in SO group (P<0.001) (Table 3 and Figure 1).
Table 3.
Ascites volume of each group (mean±SD, mL).
| Groups | 3 h (n = 8) | 6 h (n = 8) | 12 h (n = 12) |
| SO | 1.13±0.32 | 1.15±0.30 | 1.21±0.32 |
| Untreated | 4.51±1.37 | 7.06±1.91 | 14.72±2.36 |
| Emodin | 4.15±1.32 | 6.90±1.58 | 10.54±1.86b |
| Combined | 4.44±1.31 | 6.29±1.97 | 8.47±1.23bd |
| Sandostatin | 4.15±1.04 | 5.40±1.82a | 8.26±1.07bd |
P<0.05;
P<0.01 vs untreated group.
P<0.01 vs emodin group.
Figure 1.
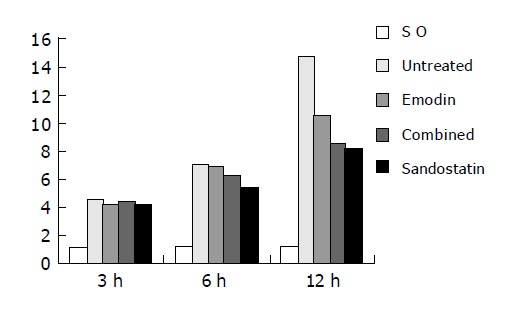
Ascites volume of each group.
Serum amylase level
The amylase levels at each time-point were lower in SO group than in all treated groups and untreated group (P<0.001). There was no difference among three treated groups (P>0.05) (Table 4 and Figure 2).
Table 4.
Serum amylase level of each group (mean±SD, U/L).
| Groups | 3 h (n = 8) | 6 h (n = 8) | 12 h (n = 12) |
| SO | 1473.38±363.80 | 1425.00±304.85 | 1489.50±176.67 |
| Untreated | 4108.50±862.24 | 5059.63±326.35 | 5668.17±547.07 |
| Emodin | 3406.00±762.16a | 4033.38±481.53b | 4399.75±577.54b |
| Combined | 3524.38±637.19a | 3941.25±435.76b | 4225.50±435.70b |
| Sandostatin | 3518.50±636.9a | 3641.00±369.02b | 4255.00±605.2b |
P<0.05;
P<0.01 vs untreated group.
Figure 2.

Serum amylase level of each group.
Serum TNF-α levelL
At each time-point, TNF-α level was significantly lower in SO group than in the untreated group and all treated groups (P<0.001). Among treated groups at 6 h, the TNF-α levels in combined and sandostatin group was markedly lower than in emodin group (P<0.01), no difference was found between the combined and sandostantin groups (P>0.05) (Table 5 and Figure 3).
Table 5.
Serum TNF-α levels of each group (mean±SD, pg/mL).
| Groups | 3 h (n = 8) | 6 h (n = 8) | 12 h (n = 12) |
| SO | 53.12±6.57 | 60.00±8.61 | 62.92±10.43 |
| Untreated | 253.25±9.69 | 353.88±23.67 | 327.50±25.62 |
| Emodin | 185.13±21.35b | 306.50±16.83b | 249.42±29.86b |
| Combined | 199.38±23.69b | 267.50±21.04bd | 264.25±40.79b |
| Sandostatin | 194.50±18.46b | 269.50±26.87bd | 232.58±23.35b |
P<0.01 vs untreated group.
P<0.01 vs emodin group.
Figure 3.
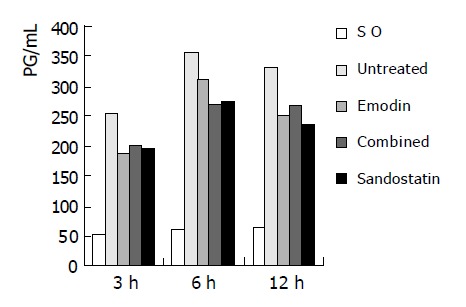
Serum TNF-α levels of each group.
Serum IL-6 level
At each time-point, IL-6 level was significantly lower in SO group than in untreated group and all treated groups (P<0.001). At 3 h, the IL-6 levels in combined and sandostatin groups was lower than in untreated group (P<0.01). At 6 and 12 h, the IL-6 levels in three treated groups were lower than those in untreated group (P<0.01). No difference was found between combined and sandostatin groups at all time-points (P>0.05) (Table 6 and Figure 4).
Table 6.
Serum IL-6 levels of each group (mean±SD, pg/mL).
| Groups | 3 h | 6 h | 12 h |
| SO | 72.75±13.26 | 65.13±13.91 | 69.08±12.89 |
| Untreated | 252.00±21.21 | 304.63±29.60 | 299.67±18.02 |
| Emodin | 236.25±25.06 | 270.63±20.85b | 259.75±18.76b |
| Combined | 217.63±22.62b | 236.75±12.09bd | 224.25±22.30bd |
| Sandostatin | 214.75±24.22b | 237.50±21.26bd | 212.00±18.78bd |
P<0.01 vs untreated group.
P<0.01 vs emodin group.
Figure 4.
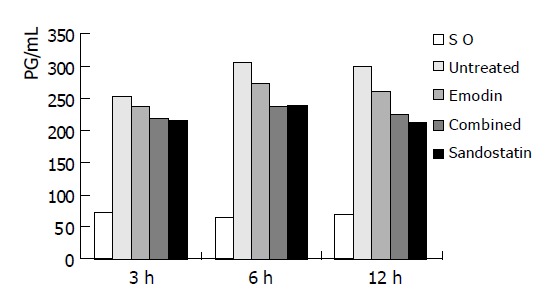
The serum IL-6 levels of each group.
Pathological lesions of pancreas
In SO group, there was no significant abnormality in pancreas at all time-points. Mild interstitial edema and inflammatory cell infiltration could be seen in some rats. But in three treated groups and the untreated group, the pathological lesions progressed gradually. However, compared with untreated group, they were obviously attenuated in three treated groups. At 12-h points, the attenuations were more remarkable.
Pathological lesion scores for pancreas
The pathological score at each time-point had no marked difference in SO group, but it was lower in the untreated group and the three treated groups (P<0.001), and was more significantly lower in treated groups than in untreated group at different time-points (P<0.05, P<0.01). There was no difference between combined and sandostatin groups at 6 and 12 h (P>0.05) (Table 7 and Figure 5).
Table 7.
Pathological lesion score for pancreas of each group (mean±SD).
| Groups | 3 h | 6 h | 12 h |
| SO | 0.50±0.53 | 0.88±0.64 | 0.83±0.48 |
| Untreated | 7.69±0.84 | 10.57±2.24 | 13.50±3.90 |
| Emodin | 6.31±1.44b | 8.75±0.97a | 10.67±0.86b |
| Combined | 6.19±0.96b | 6.94±0.73ad | 8.38±1.05bd |
| Sandostatin | 5.69±0.96b | 6.06±1.29ad | 8.17±1.64bd |
P<0.05;
P<0.01 vs untreated group.
P<0.01 vs emodin group.
Figure 5.
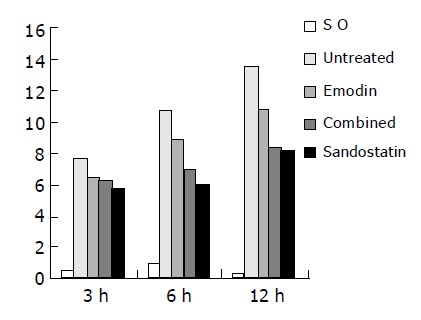
Pathological lesion score for pancreas of each group.
DISCUSSION
SAP is a life-threatening disease with a high incidence of complications. Its pathogenesis has not been completely elucidated, nevertheless, experimental and clinical studies have demonstrated that a lot of factors were involved in the local and systemic complications of SAP, such as systemic inflammatory response syndrome which could lead to multiple organ failure and pancreatic necrosis, and are the major cause of deaths. Among them, an intensive systemic inflammatory response mediated by overproduction of proinflammatory cytokines and pancreatic autodigestion by activated trypsin plays a very important role. In addition, oxidative stress, secondary infection, endotoxemia are also risk factors. So that, blocking these pathogenetic chains should be the key measures in the treatment of SAP.
Chinese traditional medicine and herbs are the precious treasure of our country, “Qingyi decoction” (Pancreas clearance soup) is an effective traditional prescription in treatment of SAP and has the advantages of a low price and a high therapeutic effect. But during the therapeutic period, patients with SAP always need fasting and gastrointestinal decompression, so oral administration is disadvantageous[7-11]. Rhubarb and Baikal skullcap root are two key herbs in “Qingyi decoction”, and emodin and baicalein are their main effective components. They can be taken in the form of an intravenous injection and their prices are low. Researches have demonstrated that both emodin and baicalein have a wide, range of pharmacodynamic effects, the effects are similar and complementary to each other in many aspects. For example, they could effectively inhibit inflammatory reaction, depress the activity of pancreatins or trypsin, antibiosis, antioxidation, scavenging free-radical and anti-thrombosis. A combination of emodin and baicalein could better block the pathogenesis of SAP on multiple levels (the pharmacodynamics of emodin and baicalein is shown in Table 8). Some studies have confirmed that intravenous injection of emodin alone can improve the prognosis and decrease the mortality of SAP in rats[3-5]. Whether combined emodin with baicalein can further enhance SAP curative effect, and cut down mortality remains to be studied.
Table 8.
| Pharmacological function | Emodin | Baicalein |
| Antibiosis | Escherichia coli, Dysentery Bacillus. Aureus staphylococcus | G+,G- bacterium, fungi, spirilla |
| Anti-inflammation | Inhibiting inflammatory cytokine release, restraining vascular permeability, inhibiting leukotrienes synthesis | Anti-histamin release. anti-arachidonic acid metabolism, restraining vascular permeability, anti-allergy |
| Immunoregulation | Enhance immunity | (-) |
| Inhibiting pancreatin activity | Strongly suppressive effect on multiple pancreatins | Only on trypsogen |
| Protecting liver | Anti-hepatofibrosis, antagonzism of liver damage by CCL4 | Anti-hepatofibrosis, |
| Protecting kidney | Inhibiting kidney compensative hypertrophy. Restraining proliferation of kidney fibroblast, preventing and curing earlier injury of kidney on rats with diabetes | (-) |
| Protecting pancreas | (+) | (-) |
| Cholagog | (+) | (-) |
| Diuresis | (+) | (+) |
| Antioxidation | (+) | (+) |
| Scavenging free-radicals | (+) | (+) |
| Anti-thrombosis | (+) | (+) |
| Circulatory system | Dilative effect on multiple blood vessel, depressing proliferation of vascular smooth muscle cell | Double effects of vasodilatation and vasoconstriction |
| Gastrointestinal tract | Double regulation of isolated intestine, anti-gastric ulcer,reducing gastric acid and pepsin, purgate | Curative effect on acute and chronic gastroenteritis |
| Anti-endotoxemia | (+) | (+) |
| Fever relieving | (–) | (+) |
“+”for positive pharmacological action; “–”for negative pharmacological action.
Our results showed that intravenous injection of emodin alone could significantly decrease the serum level of proinflammatory cytokines such as TNF-α, IL-6, and serum amylase concentration in SAP rat, and combination of emodin with baicalein further reduced the TNF-α and IL-6 levels. It indicated that the combination had better effect on blocking important factors of pathogenesis of SAP. At the same time, the combined group showed better therapeutic effect on reducing ascites volume and attenuating pancreatic pathological lesions than those of emodin alone. Sandostatin is considered to be one of the best drugs in the treatment of SAP. However, our results also showed that the therapeutic effect of a combination of emodin with bacalein was similar to that on SAP rats. Emodin and baicalein are easy to be extracted, isolated and identified, so they show an excellent prospect in the development of some new drugs for treating SAP.
In conclusion, the therapeutic effect of a combination of emodin with baicalein on SAP rats is better than emodin alone, and is worthy to be studied further.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30171167
References
- 1.Zhang XP, Li ZF. General situation in pharmacological studies on Emodin. Zhongguo Yaolixue Tongbao. 2003;19:851–854. [Google Scholar]
- 2.Zhang XP, Li ZF, Liu XG. Statusquo in the pharmacological studies on Baicalein. Zhongguo Yaolixue Tongbao. 2001;17:711–713. [Google Scholar]
- 3.Wu JX, Xu JY, Yuan YZ. Effects and mechanism of emodin and sandostatin on pancreatic ischemia in acute haemorrhagic necrotizing pancreatitis. Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:356–359. [PubMed] [Google Scholar]
- 4.Wu JX, Xu JY, Yuan YZ. Effectes of emodin and sandostatin on eicosanoid metabolism in acute hamorrhagic necrotizing pancreatitis in rats. Zhonghua Shiyan Waike Zazhi. 1997;14:215–216. [Google Scholar]
- 5.Wu JX, Xu JY, Yuan YZ. Effect of emodin and sandostatin on metabolism of eicosanoids in acute necrotizing pancreatitis. World J Gastroenterol. 2000;6:293–294. doi: 10.3748/wjg.v6.i2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spormann H, Sokolowski A, Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Pathol Res Pract. 1989;184:507–513. doi: 10.1016/S0344-0338(89)80143-8. [DOI] [PubMed] [Google Scholar]
- 7.Li YY, Gao ZF, Dui DH. Therapeutic effect of qingyi decoction and tetrandrine in treating severe acute pancreatitis in miniature pigs and serum drug level determination. Zhongguo Zhongxiyi Jiehe Zazhi. 2003;23:832–836. [PubMed] [Google Scholar]
- 8.Wen QP, Chen HL, Guan FL. Effect of qingyi decoction on rats with acute lung injury caused by severe acute pancreatitis. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 2003;9:302–306. [Google Scholar]
- 9.Yao GQ, Wu XZ. Clinical study of treatment of severe acut pancreatitis with qingyi decoction. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 1997;3:244–246. [Google Scholar]
- 10.Wu CT, Li ZL, Huang XC, Zhang ZL. Effect of Chinese medicine “QingYiTang” and bifidobacterium mixture on intestinal bacterial translocation following acute necrotizing pancceatitis. Shijie Huanren Xiaohua Zazhi. 1999;7:525–528. [Google Scholar]
- 11.Li JJ, Yang XJ, Wei MX. The effect of Qingyitang on gastrointestinal motility in rats with acute Pancreatitis. Nanjing Yikedaxue Xuebao. 2002;22:223–225. [Google Scholar]
- 12.Miyamoto K, Katsuragi T, Abdu P, Furukawa T. Effects of baicalein on prostanoid generation from the lung and contractile responses of the trachea in guinea pig. Am J Chin Med. 1997;25:37–50. doi: 10.1142/S0192415X9700007X. [DOI] [PubMed] [Google Scholar]
- 13.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 14.Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- 15.Gao D, Tawa R, Masaki H, Okano Y, Sakurai H. Protective effects of baicalein against cell damage by reactive oxygen species. Chem Pharm Bull (Tokyo) 1998;46:1383–1387. doi: 10.1248/cpb.46.1383. [DOI] [PubMed] [Google Scholar]
- 16.Shao ZH, Li CQ, Vanden Hoek TL, Becker LB, Schumacker PT, Wu JA, Attele AS, Yuan CS. Extract from Scutellaria baicalensis Georgi attenuates oxidant stress in cardiomyocytes. J Mol Cell Cardiol. 1999;31:1885–1895. doi: 10.1006/jmcc.1999.1021. [DOI] [PubMed] [Google Scholar]
- 17.Lee MJ, Wang CJ, Tsai YY, Hwang JM, Lin WL, Tseng TH, Chu CY. Inhibitory effect of 12-O-tetradecanoylphorbol-13-acetate-caused tumor promotion in benzo[a]pyrene-initiated CD-1 mouse skin by baicalein. Nutr Cancer. 1999;34:185–191. doi: 10.1207/S15327914NC3402_9. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Lin CC, Yang JJ, Namba T, Hattori M. Anti-inflammatory effects of emodin from ventilago leiocarpa. Am J Chin Med. 1996;24:139–142. doi: 10.1142/S0192415X96000189. [DOI] [PubMed] [Google Scholar]
- 19.Qi H. Anti-inflammatory effects of emodin. Zhongcaoyao Zazhi. 1995;30:522–524. [Google Scholar]
- 20.Goel RK, Das Gupta G, Ram SN, Pandey VB. Antiulcerogenic and anti-inflammatory effects of emodin, isolated from Rhamnus triquerta wall. Indian J Exp Biol. 1991;29:230–232. [PubMed] [Google Scholar]
- 21.Jiao JJ, Wen FH, Hu P. Effect of emodin on the synthesis of LTB4 by peritoneal mecrophages in rats. Tianjing Yikedaxue Xuebao. 1996;2:11–13. [Google Scholar]
- 22.Kuo YC, Tsai WJ, Meng HC, Chen WP, Yang LY, Lin CY. Immune reponses in human mesangial cells regulated by emodin from Polygonum hypoleucum Ohwi. Life Sci. 2001;68:1271–1286. doi: 10.1016/s0024-3205(00)01033-x. [DOI] [PubMed] [Google Scholar]
- 23.Kuo YC, Meng HC, Tsai WJ. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res. 2001;50:73–82. doi: 10.1007/s000110050727. [DOI] [PubMed] [Google Scholar]
- 24.Wang WJ, Wu XZ, Yao Z, Li HQ. The influence of emodin and danshensu on monocyte’s secretion of inflammatory cytokines. Zhongguo Mianyixue Zazhi. 1995;11:370–372. [Google Scholar]
- 25.Zhan YT, Wei HS, Wang ZR, Huang X, Xu QF, Li DG. Experimental study on the protective effect of emodin on rat’s liver injury induced by CCl4. Zhongguo Zhongyiyao Keji. 2000;7:30–31. [Google Scholar]
- 26.Lin CC, Chang CH, Yang JJ, Namba T, Hattori M. Hepatoprotective effects of emodin from Ventilago leiocarpa. J Ethnopharmacol. 1996;52:107–111. doi: 10.1016/0378-8741(96)01397-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhan Y, Li D, Wei H, Wang Z, Huang X, Xu Q, Lu H. Emodin on hepatic fibrosis in rats. Chin Med J (Engl) 2000;113:599–601. [PubMed] [Google Scholar]
- 28.Zhan YT, Li DG, Wei HS, Wang ZR, Huang X, Li ZD, Xu QF, Lu HM. Effect of emodin on development of hepatic fibrosis in rats. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 2000;20:276–278. [PubMed] [Google Scholar]
- 29.Liu GX, Ye RG, Tan ZM, Zhong WQ, Yang YM, Zhang GQ, Fang JA. Effect of emosin on fibroblast in lupus nephritis. Zhongguo Shiyan Linchuang Mianyixue Zazhi. 1999;11:24–27. [Google Scholar]
- 30.Zhan Y, Wei H, Wang Z, Huang X, Xu Q, Li D, Lu H. Effects of emodin on hepatic fibrosis in rats. Zhonghua Ganzangbing Zazhi. 2001;9:235–236. [PubMed] [Google Scholar]
- 31.Liu GX, Ye RG, Tan ZM, Zhong WQ, Yang YM, Zhang GQ, Fang JA. Effect of emodin on fibroblasts in lupus nephritis. Zhongguo Zhongxiyi jiehe Zazhi. 2000;20:196–198. [PubMed] [Google Scholar]
- 32.Ning Y, Wang J, Qu S. Effect of emodin on human kidney fibroblast proliferation. Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:105–106. [PubMed] [Google Scholar]
- 33.Yang JW, Li LS, Hu WX, Xu RJ. Inhibitory effect of emodin on compensatory renal hypertrophy in rats. Zhongguo Yaolixue Tongbao. 1994;10:224–227. [Google Scholar]
- 34.Jin ZH, Ma DL, Lin XZ. Study on effect of emodin on the isolated intestinal smooth muscle of guinea pigs. Zhongguo Zhongxiyi Jiehe Zazhi. 1994;14:429–431. [PubMed] [Google Scholar]
- 35.Zhen F, Li LS, Liu ZH, Zhou H, Liang LQ. The effects of emodin on cellular proliferation and c-myc prctooncogene and TGF-b gene expressions in renal tubular cells. Zhongguo Yaolixue Tongbao. 1994;10:375–378. [Google Scholar]
- 36.Dai CS, Liu ZH, Chen HP, Zhou H, Wang JP, Li LS. Combined tretment of triptolide and emodin inhibits the progression of anti-GBM nephritis in rats. Shenzangbing Yu Touxi Shenyizhi Zazhi. 2000;9:117–123. [Google Scholar]
- 37.Nakamura N, Hayasaka S, Zhang XY, Nagaki Y, Matsumoto M, Hayasaka Y, Terasawa K. Effects of baicalin, baicalein, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kappab binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp Eye Res. 2003;77:195–202. doi: 10.1016/s0014-4835(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 38.Shen YC, Chiou WF, Chou YC, Chen CF. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465:171–181. doi: 10.1016/s0014-2999(03)01378-5. [DOI] [PubMed] [Google Scholar]
- 39.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 40.Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, et al. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H999–H1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- 41.Nakahata N, Kutsuwa M, Kyo R, Kubo M, Hayashi K, Ohizumi Y. Analysis of inhibitory effects of scutellariae radix and baicalein on prostaglandin E2 production in rat C6 glioma cells. Am J Chin Med. 1998;26:311–323. doi: 10.1142/S0192415X9800035X. [DOI] [PubMed] [Google Scholar]
- 42.Butenko IG, Gladtchenko SV, Galushko SV. Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesis in vitro by flavonoid baicalein from Scutellaria baicalensis georgy roots. Agents Actions. 1993;39 Spec No:C49–C51. doi: 10.1007/BF01972717. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto K, Katsuragi T, Abdu P, Furukawa T. Effects of baicalein on prostanoid generation from the lung and contractile responses of the trachea in guinea pig. Am J Chin Med. 1997;25:37–50. doi: 10.1142/S0192415X9700007X. [DOI] [PubMed] [Google Scholar]
- 44.Inoue T, Jackson EK. Strong antiproliferative effects of baicalein in cultured rat hepatic stellate cells. Eur J Pharmacol. 1999;378:129–135. doi: 10.1016/s0014-2999(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 45.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 46.Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Bichim Biophys Acta. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- 47.Gao D, Tawa R, Masaki H, Okano Y, Sakurai H. Protective effects of baicalein against cell damage by reactive oxygen species. Chem Pharm Bull. 1998;46:1383–1387. doi: 10.1248/cpb.46.1383. [DOI] [PubMed] [Google Scholar]
- 48.Shao ZH, Li CQ, Vanden Hoek TL, Becker LB, Schumacker PT, Wu JA, Attele AS, Yuan CS. Extract from Scutellaria baicalensis Georgi attenuates oxidant stress in cardiomyocytes. J Mol Cell Cardiol. 1999;31:1885–1895. doi: 10.1006/jmcc.1999.1021. [DOI] [PubMed] [Google Scholar]
- 49.Kimura Y, Yokoi K, Matsushita N, Okuda H. Effects of flavonoids isolated from scutellariae radix on the production of tissue-type plasminogen activator and plasminogen activator inhibitor-1 induced by thrombin and thrombin receptor agonist peptide in cultured human umbilical vein endothelial cells. J Pharm Pharmacol. 1997;49:816–822. doi: 10.1111/j.2042-7158.1997.tb06119.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen ZY, Su YL, Lau CW, Law WI, Huang Y. Endothelium-dependent contraction and direct relaxation induced by baicalein in rat mesenteric artery. Eur J Pharmacol. 1999;374:41–47. doi: 10.1016/s0014-2999(99)00291-5. [DOI] [PubMed] [Google Scholar]
- 51.Huang HC, Wang HR, Hsieh LM. Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol. 1994;251:91–93. doi: 10.1016/0014-2999(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 52.Kimura Y, Matsushita N, Okuda H. Effects of baicalein isolated from Scutellaria baicalensis on interleukin 1 beta- and tumor necrosis factor alpha-induced adhesion molecule expression in cultured human umbilical vein endothelial cells. J Ethnopharmacol. 1997;57:63–67. doi: 10.1016/s0378-8741(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 53.Kimura Y, Matsushita N, Yokoi-Hayashi K, Okuda H. Effects of baicalein isolated from Scutellaria baicalensis Radix on adhesion molecule expression induced by thrombin and thrombin receptor agonist peptide in cultured human umbilical vein endothelial cells. Planta Med. 2001;67:331–334. doi: 10.1055/s-2001-14328. [DOI] [PubMed] [Google Scholar]


