Abstract
AIM: To explore the expression of Sp1 in gastric carcinoma as well as its association with other clinicopathologic features, and to evaluate the role of Sp1 as a prognostic indicator of gastric carcinoma.
METHODS: By using immunohistochemistry, we examined the Sp1 expression patterns in 65 cases of human gastric cancer, and 40 normal gastric mucosa specimens. Simultaneously, the correlation between Sp1 expression and clinical outcome or clinicopathologic features was investigated.
RESULTS: The percentage of Sp1 expression was 12.5% (5/40) in normal gastric mucosa, and the Sp1 protein was mainly expressed in the nuclei of cells located in the mucous neck region. In sharp contrast, strong Sp1 expression was detected in tumor cells, whereas no or faint Sp1 staining was detected in stromal cells and normal glandular cells surrounding the tumors. The expression rate of Sp1 in gastric cancer lesions was 53.85% (35/65). The medium survival duration in patients who had a tumor with negative, weak and strong Sp1 expressions was 1700, 1560 and 1026 d, respectively (P<0.05). Sp1 protein expression was closely related to the depth of tumor infiltration (χ2 = 13.223, P<0.01) and TNM stage (χ2 = 11.009, P<0.05), but had no relationship with the number of lymph nodes and Lauren’s classification (P>0.05). Cox regression model for multivariate analysis revealed that high Sp1 expression (P<0.05) and advanced stage (P<0.01) were independent predictors of poor survival.
CONCLUSION: Normal and malignant gastric tissues have unique Sp1 expression patterns. Sp1 might serve as an independent prognostic factor, by influencing the tumor infiltration and progression.
Keywords: Sp1, Gastric carcinoma
INTRODUCTION
Gastric carcinoma is currently one of the leading causes of cancer-related death in mainland of China. Despite improvement in early diagnosis, surgical technique and chemotherapy, the majority of gastric cancer patients die of the physiological effects of local recurrence and/or distant metastasis[1]. The overall 5-year survival rate is 30-40%. Studies over the past half century have tried to explore the mechanism of tumor metastasis on the histopathologic, cytologic and molecular biology[2-4]. Although a few critical factors such as TNM stage have been indicated to be prognostic parameters, the precise molecular parameters to predict the risk of metastasis and evaluate the outcome of gastric cancer patients remain to be defined[5,6].
The aggressive nature of gastric cancer is related to various abnormalities in oncogenes, tumor suppressor genes, growth factors and their receptors, which in turn confer growth advantages to gastric cancer cells. Increasing evidence suggests that such molecular changes are regulated by a plethora of external and internal factors[7,8]. Sp1 is a well-characterized, sequence-specific, DNA-binding protein that is important in the transcription of many cellular and viral genes containing GC boxes in their promoters[9]. Previous studies have shown that abnormal Sp1 activation might augment the growth and metastatic potential of tumor cells through over-expression of many Sp1 downstream genes. The role of Sp1 as an essential transcription factor for many genes regulating cell growth, angiogenesis and survival has been proved in pancreatic cancer[10,11]. Yet, the expression of Sp1 and its impact on the outcome of gastric cancer patients are still unknown.
In the present study, we examined Sp1 expression in human gastric cancer by using immunohistochemistry, and studied its relationship with the length of survival and clinicopathologic features.
MATERIALS AND METHODS
Patients and specimens
Sixty-five patients (45 men and 20 women, medium age 65 years) with gastric cancer undergoing radical gastrectomy (D2 or D3) in the Department of Surgery, Rui Jin Hospital, from March 1997 to September 2001, were enrolled in this study. The eligibility criteria were histologically proven gastric adenocarcinoma, no hematogenous metastatic lesions, no previous systemic chemotherapy before operation, with well-documented clinical histories and follow-up information. Distant peritoneal metastatic foci, fixed lymph nodes with a positive frozen biopsy in nodes around the middle coliac artery and/or para-aortic nodes, or involvement of the rectal shelf were considered as distant metastases and were excluded from the study.
All of the patients were observed and followed up in the same institute. At the end of March 2004, 42 patients were still alive, whereas 23 patients died of tumor recurrence and/or distant metastasis. Patients who were killed in accidents, or died of surgical-related postoperative complications as well as other cancers were excluded. All of the resected primary tumors and regional lymph nodes were histopathologically evaluated by hematoxylin and eosin staining according to the 5th UICC TNM classification published in 1997[12]. Forty normal gastric tissue specimens obtained from patients who underwent partial gastrectomy for benign gastric diseases were also collected in this study.
Immunohistochemistry
Formalin-fixed gastric cancer specimens were embedded in paraffin, and 4-μm thick sections were obtained. These sections were mounted on salinized glass slides and heated at 60 °C for 30 min. After deparaffinization in xylene, the sections were rehydrated in graded alcohol, and washed in water. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in methanol for 30 min at room temperature. Antigen retrieval was accomplished by 1 mmol/L EDTA solution. After being washed with phosphate-buffered saline (PBS) and exposed to 10% normal goat serum for 10 min to reduce non-specific binding, the slides were incubated for 12 h with rabbit monoclonal antibody against Sp1 (Santa Cruz Biotechnology, Inc., USA, Code No.: SC-59), which was diluted at 1:200 in PBS. Primary antibodies were visualized with an Envision system (DAKO, Denmark, Code No.: K4003). After further being washed with PBS, slides were incubated with substrate diaminobenzidine and hydrogen peroxide for 10 min. Finally, sections were counterstained with hematoxylin[13].
The specificity of Sp1 staining was confirmed using parallel negative control sections, which were processed immunohistochemically after the primary antibody was replaced with PBS, and positive control sections from a colonic adenocarcinoma previously were shown to express high levels of Sp1 by immunohistochemistry. Simultaneously, the samples from normal gastric mucosa were also stained according to the procedures described above.
Evaluation of immunohistochemical staining
In each specimen, immunoreactivity was examined under light microscopy and scored independently by two assessors who were blinded to patient outcomes. A positive reaction was indicated by reddish-brown precipitates in the nuclei. Only nuclear staining was considered as positive reaction. In scoring the sections, an assessment of the proportion of cell staining and the staining intensity was made. Specifically, the percentage of positive cells was divided into five grades (percentage scores): <10% (0), 10-25% (1), 25-50% (2), 50-75% (3) and >75% (4). The intensity of staining was divided into four grades (intensity scores): no staining (0), weak staining (1), moderate staining (2) and strong staining (3). Sp1 staining positivity was determined by the following formula: overall score = percentage score×intensity score. The overall score ≤3 was defined as negative, 3-6 as weak positive, and >6 as strong positive[14,15]. The concordance between the blinded reviewers was 93.3% (98/105). In the seven cases of discordance, a final evaluation was determined by consensus after reexamination.
Patient observation and follow-up
Postoperatively, the patients received chemotherapy with LV/FP (5-FU+LV+CDDP) or FM (5-FU+LV+MMC) regimen for at least 6 mo during the period of follow-up[15]. The selection of chemotherapeutic regimen was based on the histologic type and cancer stage, individually. The patients were reevaluated regularly with a physical examination and biochemical laboratory studies. Image diagnostic assessment, including computed tomography and/or ultrasonography was carried out every 6 mo.
All the enrolled patients had well-documented clinical histories and follow-up information. The epidemiological information including the age, gender and clinicopathological data including tumor type, TNM stage as well as Lauren’s classification were registered. For dead patients, the precise date and cause of death were recorded[16].
Duration of survival was defined as the time from the date of operation to the time of death. Patients who were still alive would be censored until March 31, 2004. The average follow-up period for the 65 patients was 1472 d for all patients and 1787 d for patients alive in March 2004 (range 619-2478 d). The overall survival rate was 64.6% (42/65).
Statistical analysis
Two-tailed χ2 test was performed to determine the significance of the difference between Sp1 positivity and other variables. Survival curve was calculated with the Kaplan-Meier method. Log-rank test was used to assess the statistical differences in survival. For multivariate analysis, the Cox regression model was used. In all of the tests, probability values from two-tailed test less than 0.05 were considered statistically significant. SPSS 11.0 statistic software for Windows was used for analyses.
RESULTS
Sp1 expression in human gastric cancer tissues
Expression of Sp1 in human gastric cancer tissues and its localization patterns were evaluated immunohistochemically. Moderate to strong Sp1 staining was seen in the nuclei of tumor cells of various types of gastric cancer, whereas no or weak Sp1 staining was detected in stromal cells and normal glandular cells surrounding the tumor lesions (Figure 1A). Of the 65 patients, 35 (53.85%) had Sp1 nuclear staining, while 30 (46.15%) were negative.
Figure 1.
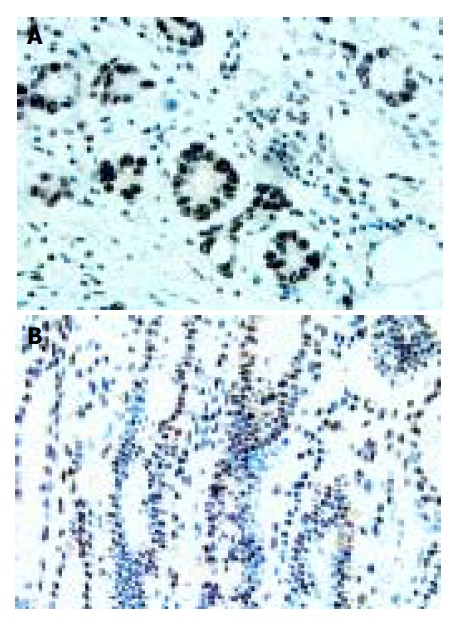
Sp1 protein expression and its localization in human gastric cancer tissue (A) and in normal human gastric tissue (B), IHC staining ×400 (A), ×200 (B).
According to the overall Sp1 staining score based on percentage and intensity, the weak and strong staining gastric cancer cases accounted for 13.85% (9/65) and 37.68% (26/65), respectively. In the 13 cases of early gastric cancer, the percentage of Sp1 expression was 15.38% (2/13), and most of them were weak staining.
Sp1 expression in normal human gastric tissues
In most of the normal gastric mucosae, no or faint nuclear staining of Sp1 was shown. Positive staining was predominantly observed in the nuclei of cells localized in the mucous neck region. There was no Sp1 protein expression in the cells of the glandular epithelial cells or cells located towards the gastric pit (foveolar) (Figure 1B). The overall percentage of Sp1 expression was only 12.5% (5/40) in normal human gastric mucosa.
Relationship between Sp1 expression and clinicopathologic features
The relationship between Sp1 expression and clinicopathologic features is summarized in Table 1. Sp1 expression was more frequently found in tumors with serosal invasion than in those without serosal invasion (χ2 = 13.223, P<0.01). Sp1 expression was also closely related to advanced TNM stage (χ2 = 11.009, P<0.05). However, no significant correlation was found between Sp1 expression and age at surgery, gender, tumor location, number of metastatic lymph nodes and Lauren’s classification (P>0.05).
Table 1.
Sp1 expression in human gastric cancer tissues and its relationship with clinicopathologic features.
| Clinicopathologicalparameters | Sp1 negativen = 30 | Sp1 weakn = 9 | Sp1 strongn = 26 | χ2 | P |
| Age (yr) | |||||
| ≥60 | 15 | 3 | 19 | 5.396 | 0.067 |
| <60 | 15 | 6 | 7 | ||
| Gender | |||||
| Male | 18 | 6 | 21 | 2.583 | 0.240 |
| Female | 12 | 3 | 5 | ||
| Location | |||||
| Upper third | 6 | 2 | 9 | 4.660 | 0.324 |
| Middle third | 3 | 2 | 6 | ||
| Lower third | 21 | 5 | 11 | ||
| Histopathologic type | |||||
| Well differentiated | 6 | 4 | 9 | 2.607 | 0.272 |
| Poorly differentiated | 24 | 5 | 17 | ||
| Depth of infiltration | |||||
| T-12 | 19 | 4 | 4 | 13.223 | 0.001 |
| T-34 | 11 | 5 | 22 | ||
| LN metastasis | |||||
| No | 19 | 5 | 9 | 4.819 | 0.306 |
| ≤6 | 6 | 2 | 10 | ||
| >6 | 5 | 2 | 7 | ||
| TNM stage | |||||
| I | 16 | 3 | 3 | 11.009 | 0.026 |
| II, III | 10 | 4 | 17 | ||
| IV | 4 | 2 | 6 | ||
| Lauren's classification | |||||
| Intestinal type | 9 | 4 | 10 | 0.811 | 0.667 |
| Diffuse type | 21 | 5 | 16 |
Sp1 immunohistochemical staining revealed that 13 of 19 well-differentiated gastric cancer tissues (including papillary adenocarcinoma, tubular adenocarcinoma and moderately differentiated adenocarcinoma) and 22 of 46 poorly differentiated gastric cancer tissues (including poorly differentiated adenocarcinoma, signet ring cell carcinoma and mucous adenocarcinoma) were positive. There was no relationship between Sp1 expression and histopathologic differentiation.
Sp1 protein expression and survival in gastric cancer patients
The median survival duration in patients who had a tumor with negative, weak and strong Sp1 expressions was 1700, 1560 and 1026 d, respectively. The elevated Sp1 expression was associated with a poor tumor prognosis (P<0.05, Figure 2).
Figure 2.
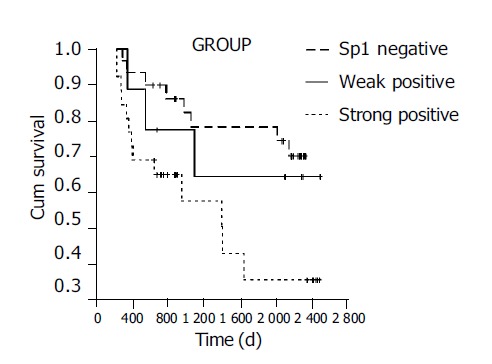
Correlation of Sp1 expression with long-term survival in gastric cancer patients.
Sp1 expression, age at surgery, gender, histologic differentiation, depth of infiltration, number of metastatic lymph nodes, TNM stage and Lauren’s classification were entered into a Cox regression model for multivariate analysis. High Sp1 expression (P<0.05) and advanced stage (P<0.01) were independent predictors of poor survival. Age at surgery, gender, histologic differentiation, depth of infiltration, number of metastatic lymph nodes and Lauren’s classification did not have a statistically significant effect on survival in multivariate analysis.
DISCUSSION
Metastasis is the most devastating aspect of gastric cancer. In fact, at the time of diagnosis, patients usually had locally advanced diseases or metastasis involving the lymph nodes, liver or peritoneum[17]. Understanding the critical determinants of cancer, metastasis is helpful in designing potential preventive and therapeutic strategies. Studies over the past several decades have indicated that the process of cancer metastasis consists of a series of conceptual and sequential steps[18]. Malignant neoplasm consists of multiple cell populations that exhibit a wide range of biologic characteristics, including antigenicity, chemosensitivity, growth rate, karyotype as well as metastatic potential[19,20]. The development of multiple growth signaling pathways could render tumor cells a tremendous growth advantage[2,19,20]. A number of molecular events are being recognized to play a part in metastatic process.
Sp1 is a sequence-specific DNA-binding protein, which is important in the transcription of many cellular and viral genes that contain GC elements within the proximal region of their promoter[21]. Recently, additional transcription factors such as Sp2, Sp3 and Sp4, which are similar to Sp1 in their structures and transcriptional properties, have been cloned[22,23]. Sp1 is essential for many genes that regulate the cell survival, growth and angiogenesis. Earlier studies have established that Sp1 protein can regulate many tumor-related genes, including FGFR1, insulin-like growth factor receptor1, insulin-like growth factor-binding protein 2, VEGF and thymidine kinase[24,25]. The relevance of these factors to tumor growth and metastasis has been evaluated. Abnormal Sp1 protein activation and expression might up-regulate the production of these genes, thus creating a microenvironment, which favors tumor cell growth, metastasis and angiogenesis. Sp1 signaling pathway may contribute to tumor development and progression.
It was reported that Sp1 involved apoptosis by altering the expression and function of multiple apoptosis-related proteins, including Bcl-2 and Bax[26].
The mechanism underlying in different levels of Sp1 is currently unknown. Investigations indicated that Sp1 expression was influenced by many stimuli, and varied in different tumor origins. Kumar and Butler[27] revealed that the DNA-binding activity of Sp1 was significantly higher in human epithelium-derived cancer than in human skin papilloma. A recent study showed that Sp1 activity could be modulated by stress factors such as hypoxia, acidosis and over-expression of free radicals, thus activating the p42/p42 mitogen-activated protein kinase and c-JunNH2-terminal kinase-related signal pathways[28]. It may also involve many other cellular factors, including the functional status of oncogenes and tumor suppressor genes.
In agreement with a previous study, we did not find strong expression of Sp1 in nuclei of normal gastric mucosa cells. The normal gastric mucosa not displayed or little staining for Sp1, and the positive cells were located in the mucous neck region in normal gastric mucosa, suggesting that Sp1 is mainly expressed in dividing cells, and not in differentiated cells. In contrast, our results showed that expression of Sp1 was a prominent feature of advanced gastric cancer, regardless of its histologic type. In the present study, we identified nuclear expression of Sp1 in 63.46% (33/52) of advanced gastric carcinoma cases, and 15.38% (2/13) of early gastric cancer cases. Shi et al[29], studied the relationship between constitutive Sp1 activity and constitutive expression of VEGF in human pancreatic adenocarcinomas, and found that the level of Sp1 activity was directly correlated with the VEGF promoter activity, the steady-state level of mRNA, and VEGF secretion. These results suggested that altered expression of Sp1 in human pancreatic cancer might lead to the over-expression of multiple Sp1 downstream genes, which in turn could enhance the tumor angiogenesis and contribute to the aggressive biology of human gastric cancer. In the present study, we also found that the expression of Sp1 protein was correlated with increasing gastric cancer stage and depth of invasion. There was no significant difference in Sp1 expression between tumors with more or less lymph node metastasis. These data suggest that Sp1 might contribute to the progression and dissemination of human gastric caner via other pathways except lymph node metastasis. Sp1 expression was inversely correlated with patient long-term survival, suggesting that Sp1 could serve as an independent and sensitive prognostic marker for gastric cancer.
In summary, Sp1 is aberrantly over-expressed in gastric adenocarcinoma. Sp1 expression is closely related to the advanced stage, depth of tumor infiltration as well as poor outcome of the patients. A further understanding of the molecular basis of Sp1 will have functional implications in suppressing gastric cancer progression and ultimately lead to the design of new therapeutic strategies. On the basis of this study, we propose that increasing Sp1 expression may augment the metastatic potential of tumor cells, and may be a reliable tumor progression marker and/or effective therapeutic target for gastric cancer.
ACKNOWLEDGMENTS
We are grateful to all of the individuals described here for their contribution to this study.
Footnotes
Supported by the “211” Project sponsored by Ministry of Education, China
References
- 1.Lu JB, Sun XB, Dai DX, Zhu SK, Chang QL, Liu SZ, Duan WJ. Epidemiology of gastroenterologic cancer in Henan Province, China. World J Gastroenterol. 2003;9:2400–2403. doi: 10.3748/wjg.v9.i11.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 3.Ajisaka H, Yonemura Y, Miwa K. Correlation of lymph node metastases and expression of matrix metalloproteinase-7 in patients with gastric cancer. Hepatogastroenterology. 2004;51:900–905. [PubMed] [Google Scholar]
- 4.Miyachi K, Sasaki K, Onodera S, Taguchi T, Nagamachi M, Kaneko H, Sunagawa M. Correlation between survivin mRNA expression and lymph node metastasis in gastric cancer. Gastric Cancer. 2003;6:217–224. doi: 10.1007/s10120-003-0255-2. [DOI] [PubMed] [Google Scholar]
- 5.Klein Kranenbarg E, Hermans J, van Krieken JH, van de Velde CJ. Evaluation of the 5th edition of the TNM classification for gastric cancer: improved prognostic value. Br J Cancer. 2001;84:64–71. doi: 10.1054/bjoc.2000.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fondevila C, Metges JP, Fuster J, Grau JJ, Palacín A, Castells A, Volant A, Pera M. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer. 2004;90:206–215. doi: 10.1038/sj.bjc.6601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JW, Kim H, Kim KH. Expression of Ku70 and Ku80 mediated by NF-kappa B and cyclooxygenase-2 is related to proliferation of human gastric cancer cells. J Biol Chem. 2002;277:46093–46100. doi: 10.1074/jbc.M206603200. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Chen S. Screening and identification of gastric adenocarcinoma metastasis-related genes using cDNA microarray coupled to FDD-PCR. J Cancer Res Clin Oncol. 2002;128:547–553. doi: 10.1007/s00432-002-0379-5. [DOI] [PubMed] [Google Scholar]
- 9.Roder K, Kim KH, Sul HS. Induction of murine H-rev107 gene expression by growth arrest and histone acetylation: involvement of an Sp1/Sp3-binding GC-box. Biochem Biophys Res Commun. 2002;294:63–70. doi: 10.1016/S0006-291X(02)00440-0. [DOI] [PubMed] [Google Scholar]
- 10.Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. doi: 10.1158/0008-5472.can-03-1945. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S, Venkatasubbarao K, Li S, Freeman JW. Requirement of a specific Sp1 site for histone deacetylase-mediated repression of transforming growth factor beta Type II receptor expression in human pancreatic cancer cells. Cancer Res. 2003;63:2624–2630. [PubMed] [Google Scholar]
- 12.de Manzoni G, Verlato G, Roviello F, Morgagni P, Di Leo A, Saragoni L, Marrelli D, Kurihara H, Pasini F. The new TNM classification of lymph node metastasis minimises stage migration problems in gastric cancer patients. Br J Cancer. 2002;87:171–174. doi: 10.1038/sj.bjc.6600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res. 2003;9:6371–6380. [PubMed] [Google Scholar]
- 14.Chen F, Zhang F, Rao J, Studzinski GP. Ectopic expression of truncated Sp1 transcription factor prolongs the S phase and reduces the growth rate. Anticancer Res. 2000;20:661–667. [PubMed] [Google Scholar]
- 15.Van Cutsem E, Haller D, Ohtsu A. The role of chemotherapy in the current treatment of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:17–22. doi: 10.1007/s10120-002-0219-y. [DOI] [PubMed] [Google Scholar]
- 16.Ruo L, Coit DG, Brennan MF, Guillem JG. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671–675. doi: 10.1016/s1091-255x(02)00045-8. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi O, Sugiyama Y, Cho H, Tsuburaya A, Sairenji M, Motohashi H, Yoshikawa T. Clinical and pathological study of gastric cancer with ovarian metastasis. Int J Clin Oncol. 2003;8:67–71. doi: 10.1007/s101470300012. [DOI] [PubMed] [Google Scholar]
- 18.Yokota J, Nishioka M, Tani M, Kohno T. Genetic alterations responsible for metastatic phenotypes of lung cancer cells. Clin Exp Metastasis. 2003;20:189–193. doi: 10.1023/a:1022978932215. [DOI] [PubMed] [Google Scholar]
- 19.Yasui W, Oue N, Ito R, Kuraoka K, Nakayama H. Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci. 2004;95:385–392. doi: 10.1111/j.1349-7006.2004.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koide N, Nishio A, Hiraguri M, Shimada K, Shimozawa N, Hanazaki K, Kajikawa S, Adachi W, Amano J. Cell proliferation, apoptosis and angiogenesis in gastric cancer and its hepatic metastases. Hepatogastroenterology. 2002;49:869–873. [PubMed] [Google Scholar]
- 21.Suzuki T, Muto S, Miyamoto S, Aizawa K, Horikoshi M, Nagai R. Functional interaction of the DNA-binding transcription factor Sp1 through its DNA-binding domain with the histone chaperone TAF-I. J Biol Chem. 2003;278:28758–28764. doi: 10.1074/jbc.M302228200. [DOI] [PubMed] [Google Scholar]
- 22.Koutsodontis G, Moustakas A, Kardassis D. The role of Sp1 family members, the proximal GC-rich motifs, and the upstream enhancer region in the regulation of the human cell cycle inhibitor p21WAF-1/Cip1 gene promoter. Biochemistry. 2002;41:12771–12784. doi: 10.1021/bi026141q. [DOI] [PubMed] [Google Scholar]
- 23.Mao X, Moerman AM, Barger SW. Neuronal kappa B-binding factors consist of Sp1-related proteins. Functional implications for autoregulation of N-methyl-D-aspartate receptor-1 expression. J Biol Chem. 2002;277:44911–44919. doi: 10.1074/jbc.M204292200. [DOI] [PubMed] [Google Scholar]
- 24.Patel SG, DiMario JX. Two distal Sp1-binding cis-elements regulate fibroblast growth factor receptor 1 (FGFR1) gene expression in myoblasts. Gene. 2001;270:171–180. doi: 10.1016/s0378-1119(01)00478-4. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Chen YH, Liu TJ, Jia J, Hampson S, Shan YX, Kibler D, Wang PH. Using DNA microarray to identify Sp1 as a transcriptional regulatory element of insulin-like growth factor 1 in cardiac muscle cells. Circ Res. 2003;93:1202–1209. doi: 10.1161/01.RES.0000104085.76261.02. [DOI] [PubMed] [Google Scholar]
- 26.Trisciuoglio D, Iervolino A, Candiloro A, Fibbi G, Fanciulli M, Zangemeister-Wittke U, Zupi G, Del Bufalo D. bcl-2 induction of urokinase plasminogen activator receptor expression in human cancer cells through Sp1 activation: involvement of ERK1/ERK2 activity. J Biol Chem. 2004;279:6737–6745. doi: 10.1074/jbc.M308938200. [DOI] [PubMed] [Google Scholar]
- 27.Kumar AP, Butler AP. Enhanced Sp1 DNA-binding activity in murine keratinocyte cell lines and epidermal tumors. Cancer Lett. 1999;137:159–165. doi: 10.1016/s0304-3835(98)00351-6. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida S, Harada H, Nagai H, Fukino K, Teramoto A, Emi M. Head-to-head juxtaposition of Fas-associated phosphatase-1 (FAP-1) and c-Jun NH2-terminal kinase 3 (JNK3) genes: genomic structure and seven polymorphisms of the FAP-1 gene. J Hum Genet. 2002;47:614–619. doi: 10.1007/s100380200094. [DOI] [PubMed] [Google Scholar]
- 29.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, Xiong Q, Wang B, Li XC, Xie K. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143–4154. [PubMed] [Google Scholar]


