Abstract
AIM: The role of hepatitis B virus (HBV) genotypes on the clinical features and prognosis of patients with hepatocellular carcinoma (HCC) is currently unknown. The aim of the present study was to evaluate the distribution of HBV genotypes and their clinical relevance in Thai patients.
METHODS: HBV genotypes were determined by PCR-RFLP in stored sera of 93 asymptomatic carriers, 103 patients with chronic hepatitis, 60 patients with cirrhosis and 76 patients with HCC. The clinical data were analyzed in relation to the HBV genotype.
RESULTS: HBV genotypes C and B were predominant in Thailand, accounting for 73% and 21%, respectively. The distributions of genotypes B and C were similar in HCC patients compared to the other groups. Genotype C was significantly more common in HCC patients who were under 40 years old than genotype B (18% vs 0%, P = 0.03), but was significantly less common in patients older than 60 years (26% vs 56.5%, P = 0.01). The positive rate of hepatitis B e antigen (HBeAg) in patients with genotype C was significantly higher than that in patients with genotype B (71.6% vs 44.4%, P = 0.03 in chronic hepatitis; 56.8% vs 11.1%, P = 0.01 in cirrhosis). There were no differences between HCC patients with genotypes B and C regarding tumor staging by CLIP criteria and the overall median survival. Multivariate analyses showed that HBV genotype was not an independent prognostic factor of survival in HCC patients.
CONCLUSION: Patients with genotype C had a higher positive rate of HBeAg and exhibited earlier progression of cirrhosis and HCC than those with genotype B. However, there were no differences in the risk of developing HCC and its prognosis between patients with these genotypes.
Keywords: HBV, Genotype, Hepatocellular carcinoma
INTRODUCTION
Hepatitis B virus (HBV) infection is associated with a diverse clinical spectrum of liver damage ranging from asymptomatic carrier, chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC)[1]. HBV, a member of the hepadnaviridae, is a relaxed circular double-stranded DNA virus, and has currently been classified into eight genotypes, designed A-H based on a comparison of entire genomic sequences[2,3]. HBV genotypes appear to show varying geographic patterns in their distribution. For instance, genotypes A and D are predominant in Western countries and India, whereas genotypes B and C prevail in Southeast Asia, China and Japan. Genotype E is restricted to Africa and genotype F is found in Central and South America.
Besides the differences in geographical distribution, there is growing evidence that the viral genotypes may influence the clinical outcomes of patients with chronic HBV infection. Among Asian patients who constitute approximately 75% of HBV carriers worldwide, it has been shown that HBV genotype C is more commonly associated with severe liver diseases and the development of cirrhosis compared to genotype B[4-7]. Genotype C is also associated with a lower rate of hepatitis B e antigen (HBeAg) seroconversion and a lower response rate to alpha interferon therapy compared to genotype B[8,9]. However, the association between HBV genotype and the risk of developing HCC is still controversial[4,10-12]. In addition, the impact of HBV genotype on clinical features and prognosis of patients with HCC remains unclear. Thus, the aim of this study was to determine the role of HBV genotypes on clinical severity of patients with chronic liver disease, particularly those with HCC.
MATERIALS AND METHODS
Subjects
Serum samples were obtained from 470 patients with chronic HBV infection who had undergone long-term follow-up at Chulalongkorn Memorial Hospital (Bangkok, Thailand), and the National Blood Center, Thai Red Cross, between August 1997 and August 2003. All patients were positive for Hepatitis B s antigen (HBsAg), as determined by the use of a commercially available enzyme-linked immunosorbent assay kit (Abbott Laboratories, Chicago, IL). Of these, patients who were positive for hepatitis C virus antibody (anti-HCV) and those who had another potential cause of chronic liver disease were excluded. Patients who had previously been treated with antiviral therapy were excluded. The patients were clinically classified into four groups including asymptomatic carrier, chronic hepatitis, cirrhosis and HCC. Asymptomatic carrier was diagnosed by periodical examination of normal serum alanine aminotransferase (ALT) level for at least 1 year. Chronic hepatitis was diagnosed by the presence of prolonged elevation of serum ALT level, and confirmed by histologic examinations. The degree of hepatic inflammation and fibrosis was graded according to modified Knodell histology index[13]. Cirrhosis was diagnosed based on histologic examinations and/or imaging studies, and its severity was subsequently classified based on Child’s criteria. HCC was established by histopathology and/or a combination of mass lesions in the liver on hepatic imaging and serum alpha-fetoprotein (AFP) levels above 400 IU/mL. The staging of HCC was classified according to CLIP criteria[14].
Serum samples were collected from each patient at the time of their clinical evaluation and stored at -70 °C until further tests were performed. All patients were informed regarding the purpose of exterminating the etiologies of liver disease and their written consent were obtained. The study was approved by the Ethics Committee, Faculty of Medicine, Chulalongkorn University.
HBV-DNA extractions
DNA was extracted from 100 μL serum with proteinase-K/SDS in Tris buffer, followed by phenol/chloroform extraction and ethanol precipitation. The pellet was dissolved in 30 μL sterile water and directly subjected to PCR-based amplification.
HBV-DNA detection
HBV-DNA was amplified in an automated thermocycler (Perkin Elmer Cetus, Branchburg, NJ), using the primer sequences previously described[15]. The forward primer was P1 (nt 2823-2845: 5’-TCACCATATTCTTGGGAACAAGA); the reverse primer was P2 (nt 80-61: 5’-TTCCTGAAC-TGGAGCCACCA). The primers were located in conserved genomic regions to ensure a high sensitivity for the ampli-fication of all HBV genotypes. Two microliters of DNA sample were combined with a reaction mixture containing 20 μL of 2.5×Eppendorf MasterMix (Hamburg, Germany), 1 μmol/L P1, 1 μmol/L P2 and sterile water, in a final volume of 50 μL. PCR was performed under the following conditions: After an initial 2-min denaturation step at 94 °C, 35 cycles of amplification were performed, each including 30-s denaturation at 94 °C, 30-s annealing at 55 °C and 30-s extension at 72 °C, followed by a final 10-min extension at 72 °C. Each amplified DNA sample (10 μL) was added to a loading buffer and run on a 2% agarose gel (FMC Bioproducts, Rockland, ME) at 100 V for 60 min. The 479-bp product stained with ethidium bromide on preparation was visualized on a UV transilluminator.
PCR-RFLP analysis for genotyping
PCR products were subjected to RFLP analysis, using restriction endonuclease AvaII and DpnII (New England Biolabs, Beverly, MA) to determine the HBV genotype. Briefly, 10 μL of PCR product was mixed with 1.5 μL of 10×buffer, 3 μL of sterile water and 0.5 μL (5U) of AvaII and DpnII, respectively, in separate reactions and incubated at 37 °C for 3.5 h. After incubation, the samples were run on a composite gel containing 2% NuSieve agarose (FMC BioProducts, Rockland, ME) and 1% standard agarose. The sizes of the RFLP products, visible under UV light as a result of prior ethidium bromide staining, served to identify the various HBV genotypes based on the polymorphism patterns[15].
Serological and virological assays
HBeAg was determined using commercially available enzyme-linked immunosorbent assay kit (Abbott Laboratories, Chicago, IL). Serum HBV-DNA level was quantified using a commercial kit (Amplicor HBV Monitor, Roche Diagnostics, Tokyo, Japan). The detection range of this assay was from 2.7 to 8.7 log genome equivalents/mL (LGE/mL).
Statistical analysis
Data were presented as percentage, mean and standard deviation. χ2 test, unpaired t test, and ANOVA were used to assess the statistical significance of the difference between groups where appropriate. Survival curves were established using the Kaplan-Meier method and differences between curves were verified using the log-rank test. Cox regression analysis was performed to identify which indepe-ndent variables would have a significant influence on the overall survival. P values below 0.05 were considered statis-tically significant. All statistical analyses were performed using SPSS 10.0 software for Windows (SPSS, Inc., Chicago, IL).
RESULTS
Distribution of HBV genotypes in patients with chronic HBV infection
Of the 470 patients enrolled in this study, HBV-DNA was detected in 332 patients (70.6%). Our data showed that the most common HBV genotypes were genotypes C and B, which were found in 243 (73.2%) and 69 (20.8%) patients, respectively. The remaining 20 cases included 11 (3.3%) with genotype A and 9 (2.7%) with unclassified genotype. The demographic and clinical data of 332 patients with different stages of chronic HBV infection are shown in Table 1. Male-to-female ratio of asymptomatic carriers was significantly lower than the other groups (P = 0.002). Mean age was significantly higher in patients with cirrhosis and HCC than in the other two groups (P = 0.001), and positive HBeAg rate was significantly higher in patients with chronic hepatitis than in other groups (P = 0.001). Although genotype C was the most common genotype in each group, no significant differences were observed with respect to the distribution of the genotypes in various stages of chronic HBV infection (P = 0.16).
Table 1.
Demographic and clinical data of 332 patients with chronic HBV infection.
| Diagnosis | n | Sex (m/f) | Age (yr) | ALT (U/L) | HBeAg positive |
Genotype |
|||
| A | B | C | U | ||||||
| Carrier | 93 | 57/36 | 30.9±10.6 | 27.5±4.5 | 42/82 (51.2) | 2 (2.2) | 16 (17.2) | 73 (78.4) | 2 (2.2) |
| CH | 103 | 84/19 | 36.2±10.1 | 157.4±103.8 | 61/92 (66.3) | 5 (4.9) | 20 (19.4) | 76 (73.8) | 2 (1.9) |
| Cirrhosis | 60 | 47/13 | 48.8±13.8 | 135.0±90.9 | 26/53 (49.1) | 2 (3.3) | 10 (16.7) | 44 (73.3) | 4 (6.7) |
| HCC | 76 | 60/16 | 54.4±12.9 | 107.8±107.4 | 15/71 (21.1) | 2 (2.6) | 23 (30.3) | 50 (65.8) | 1 (1.3) |
CH, chronic hepatitis; HCC, hepatocellular carcinoma; U, unclassified genotype. Quantitative variables are expressed as mean±SD; categorical variables are expressed as n (%).
Clinicopathologic differences between genotypes B and C in chronic hepatitis
Because the number of patients with genotype A was less, only genotypes B and C were included for further analysis of clinicopathologic differences between genotypes. As shown in Table 2, patients with genotypes B and C were comparable with respect to sex, age and total bilirubin. The rate of positive HBeAg in patients with genotype B was significantly lower than that in patients with genotype C (44.4% vs 71.6%, respectively, P = 0.03). Mean ALT level was also significantly lower in patients with genotype B than those in patients with genotype C (119.8±58.5 and 159.8±106.4 IU/L, respectively; P = 0.03), but HBV-DNA levels were comparable between them (7.25±1.74 and 7.10±1.34 LGE/mL, respectively; P = 0.78). Patients with genotype B had a lower score of both necroinflammation activity and fibrosis than those with genotype C, but the differences were not statistically significant (5.8±2.1 and 1.5±1.0 vs 6.7±2.1 and 1.8±0.9, P = 0.51 and 0.47, respectively).
Table 2.
Demographic and clinical data of patients with chronic hepatitis.
| Characteristics | Genotype B (n = 20) | Genotype C (n = 76) | P |
| Age (yr) | 35.1±9.6 | 36.4±10.3 | NS |
| Sex (male/female) | 16/4 | 65/11 | NS |
| Total bilirubin (mg/dL) | 0.8±0.2 | 0.7±0.3 | NS |
| ALT (IU/L) | 119.8±58.5 | 159.8±106.4 | 0.03 |
| HBeAg positive | 8/18 (44.4) | 53/74 (71.6) | 0.03 |
| HBV DNA (LGE/mL) | 7.25±1.74 | 7.10±1.34 | NS |
| HAI inflammation | 5.8±2.1 | 6.7±2.1 | NS |
| HAI fibrosis | 1.5±1.0 | 1.8±0.9 | NS |
Quantitative variables are expressed as mean±SD. Categorical variables are expressed as n (%). HAI inflammation, sum of necroinflammatory scores of histology activity index. HAI fibrosis, sum of fibrosis scores of histology activity index.
Clinical differences between genotypes B and C in cirrhosis
There were no significant differences in gender, total bilirubin, serum albumin, and child classification between groups of patients, as shown in Table 3. The mean age of patients with genotype B tended to be older than those with genotype C (54.7±9.2 vs 47.4±14.4 years, respectively, P = 0.06). The positive rate of HBeAg in patients with genotype B was significantly lower than that in patients with genotype C (11.1% vs 56.8%, respectively, P = 0.01). Patients with genotype B tended to have lower mean ALT and HBV-DNA levels than those with genotype C, but the difference was not statistically significant (101.5±49.0 IU/L and 6.59±1.20 LGE/mL vs 133.7±93.4 IU/L and 7.05±2.50 LGE/mL, P = 0.14 and 0.12, respectively).
Table 3.
Demographic and clinical data of patients with cirrhosis.
| Characteristics | Genotype B (n = 10) | Genotype C (n = 44) | P |
| Age (yr) | 54.7±9.2 | 47.4±14.4 | 0.06 |
| Sex (male/female) | 8/2 | 35/9 | NS |
| Total bilirubin (mg/dL) | 1.4±0.8 | 2.2±1.2 | NS |
| ALT (IU/L) | 101.5±49.0 | 133.7±93.4 | NS |
| Albumin (g/dL) | 3.7±0.4 | 3.8±0.5 | NS |
| HBeAg positive | 1/9 (11.1) | 25/44 (56.8) | 0.01 |
| HBV DNA (LGE/mL) | 6.59±1.20 | 7.05±2.50 | NS |
| Child classification (A/B/C) | 7/2/1 | 24/15/5 | NS |
Quantitative variables are expressed as mean±SD. Categorical variables are expressed as n (%).
Clinical differences between genotypes B and C in HCC
The clinical data of 73 patients with HCC were compared according to HBV genotype, as shown in Table 4. Between the two groups, there were no significant differences in gender, total bilirubin, ALT, serum albumin, AFP and HBV-DNA levels, tumor staging according to CLIP criteria. However, the mean age of patients with genotype B was significantly older than those with genotype C (61.1±9.8 vs 51.3±13.1 years, respectively, P = 0.001). Four of 22 patients (18.1%) with genotype B were positive for HBeAg, whereas 11 of 49 patients (22.4 %) with genotype C were positive for this marker, but the difference was not statistically significant (P = 0.68). When patients with HCC were stratified by age (Figure 1), none of the patients with genotype B was younger than 40 years, whereas nine patients (18 %) with genotype C were younger than 40 years (P = 0.03). On the contrary, 13 patients (56.5%) with genotype B were older than 60 years, whereas 13 patients (26 %) with genotype C were older than 60 years (P = 0.01).
Table 4.
Demographic and clinical data of patients with HCC.
| Characteristics | Genotype B (n = 23) | Genotype C (n = 50) | P |
| Age (yr) | 61.1±9.8 | 51.3±13.1 | 0.001 |
| Sex (male/female) | 20/3 | 38/12 | NS |
| Total bilirubin (mg/dL) | 2.1±2.0 | 2.9±4.9 | NS |
| ALT (IU/L) | 95.5±102.6 | 121.4±126.1 | NS |
| Albumin (g/dL) | 3.4±0.7 | 3.5±0.5 | NS |
| HBeAg positive | 4/22 (18.1) | 11/49 (22.4) | NS |
| HBV DNA (LGE/mL) | 6.71±1.62 | 6.52±2.63 | NS |
| AFP (IU/mL) | 42 485.2±95 590.9 | 42 032.6±89 382.8 | NS |
| CLIP score (0-1/2-3/4-6) | 5/7/11 | 8/25/17 | NS |
Quantitative variables are expressed as mean±SD. Categorical variables are expressed as n (%).
Figure 1.
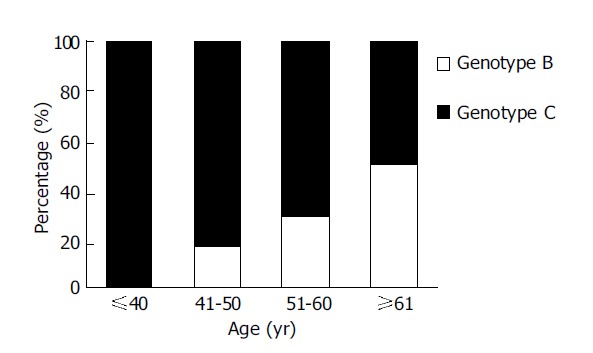
HBV genotypes B and C in 73 patients with HCC.
The Kaplan-Meier survival curves demonstrated that the overall median survival for patients with genotypes B and C were 5.5 and 7.3 mo, respectively (P = 0.81, using log-rank test) (Figure 2). For patients who were treated with any specific therapeutic modality, the median survival for the genotype B and C groups were 11.5 and 12.4 mo, respectively (P = 0.97). In the untreated cases, the median survival of the genotype B and C groups were 4.5 and 4.0 mo, respectively (P = 0.85).
Figure 2.
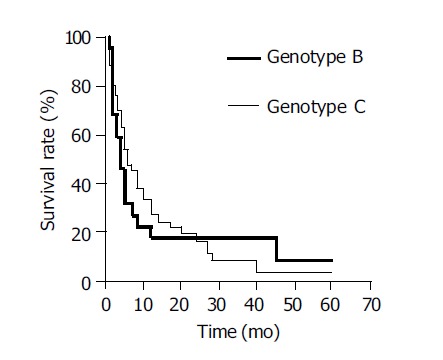
Overall survival of HCC patients with HBV genotypes B and C.
HBV genotype was entered into Cox regression analysis together with other variables that would influence prognosis. These included sex, age, HBeAg, HBV-DNA level, CLIP stage and therapy for HCC. The multivariate analyses revealed that independent unfavorable factors of overall survival included CLIP stage and lack of therapy for HCC (Table 5). However, the HBV genotype was not selected as an independent predictor of survival.
Table 5.
Multivariate analysis of unfavorable factors of survival in patients with HCC, by using Cox regression analysis.
| Factors | Risk ratio | 95%CI | P |
| CLIP stage | 4.63 | 1.70-12.62 | 0.003 |
| No therapy | 6.22 | 2.54-15.22 | 0.001 |
DISCUSSION
Identification of host and viral factors leading to severe liver damage and to the development of HCC may have important clinical implications in the management of patients with chronic HBV infection. There are now increasing data suggesting that HBV genotypes may play an important role in causing different disease profiles in chronic HBV infection. Most studies on HBV genotype and its clinical relevance have been performed in Asia and restricted to comparisons between genotypes B and C, which are the two most common HBV genotypes in this region accounting for more than 90% of cases[6,16,17]. Current available data from this region demonstrate that HBV genotype C is more commonly associated with severe liver diseases and the development of cirrhosis compared to genotype B[4-7]. In addition, patients with genotype C infection, compared to those with genotype B, are more frequently HBeAg positive and display higher HBV-DNA levels that may contribute to multiple episodes of acute flares and progression of liver disease[18]. Taken together, these data suggest that patients with genotype C have a tendency to exhibit more severe liver disease than those with genotype B.
In agreement with previous studies[17,19], our study demo-nstrated that genotype C and B were the predominant strains, accounting for approximately 75% and 20% of patients, respectively. In this respect, it would appear that the prevalence of HBV genotypes in Thailand is comparable to that reported from Japan and China[6,16], but differs from the distribution observed in Taiwan, where HBV genotype B is more common than genotype C[4,20]. Interestingly, the prevalence of genotype B and C in patients with HCC in our study was comparable to that in asymptomatic carrier, chronic hepatitis and cirrhosis. The equal distribution of genotypes B and C among various stages of chronic liver disease is consistent with previous reports conducted in Japan[6,11], but it contradicts the observations from other studies[4,7,16]. Hence, our data suggest that although genotype C is the most prevalent strain in Thailand, the risk of develo-pment of HCC may not be different between genotypes B- and C-related chronic liver disease.
The predominance of HBV genotypes B and C allows the comparison of clinical outcomes of patients who are chro-nically infected with these two HBV strains. Our results showed that the mean ages among asymptomatic carriers and chronic hepatitis were comparable between patients with genotypes B and C. However, the mean age of patients with genotype C tended to be older than those with genotype B in cirrhotic group. Interestingly, the divergence in the mean age of patients with genotypes B and C was more noticeably in those with HCC. Given that the majority of Thai patients acquire HBV infection vertically from their mothers at birth or horizontally during early childhood from carrier family members, their age would probably serve as a reasonable surrogate for the duration of HBV infection, regardless of the viral genotype. Our results also showed that patients with genotype C had a tendency of higher ALT, necroinflammatory scores and HBV-DNA levels than patients with genotype B. Moreover, patients with genotype C had a significantly higher prevalence of HBeAg positivity, particularly among patients with chronic hepatitis and cirrhosis, but the difference seemed to be disappearing upon the disease progression to HCC. Although HBeAg is a marker of active viral replication, the disappearance of HBeAg with or without seroconversion of antibody to HBeAg during the course of chronic infection does not always imply disease remission[21]. Collectively, it is reasonable to speculate that, at least in our populations, patients with genotype C have a trend for delayed HBeAg seroconversion and more prolonged necroinflammatory process causing earlier development of cirrhosis and HCC. Nonetheless, it would appear that there is no difference in the risk between patients with genotypes B and C in the progression to liver cancer.
Indeed, it is currently unclear whether a certain HBV genotype is associated with a greater risk for progression of cirrhosis to HCC. Studies from Taiwan and Japan have demonstrated an increase in HCC development among patients with HBV genotype C compared to genotype B[4,6,12,20]. Similarly, a study conducted in China has sugges-ted that genotype C may predispose to HCC, whereas geno-type B has a relatively better prognosis[16]. On the contrary, recent studies from Hong Kong and Japan have shown that there is no difference in the risk of developing HCC between patients with genotypes B and C[11,22]. Moreover, a potential correlation between HBV genotype and the age of the patients with HCC has been debated. Intriguingly, the report from northern Taiwan has observed that genotype B is associated with the development of HCC in patients younger than 35 years of age, while those with genotype C more frequently develop cancer after 50 years of age[4]. This observation, however, has not been confirmed by subse-quent studies from southern Taiwan and Japan[6,11,20]. In southern Taiwan, for example, there is no significant difference in the mean age between HCC patients with genotypes B and C[20]. By remarkable contrast, the mean age of Japanese patients with genotype B is approximately 70 years compared to 55 years of those with genotype C[6]. In the present report, the mean age of HCC patients with genotype B was significantly older than those with genotype C (61 and 51 years, respectively). Thus, the age distribution of HBV genotypes in Thai patients with HCC seems to correspond with the report from Japan, but differs from those studies from Taiwan.
The molecular virological factors responsible for this discrepancy among countries remain largely unknown. It has been postulated that the difference in the mean age between Taiwanese and Japanese patients with HCC may be partially influenced by the divergence of HBV subtypes distributed among different geographic areas[23]. Recently, two subtypes of HBV genotype B, namely Ba and Bj, have been identified based on the phylogenetic analysis. It has shown that genotype Ba consists of the recombination with the precore/core region originating from genotype C, whereas genotype Bj does not[24]. Genotype Bj is exclusively found in Japan, while genotype Ba is ubiquitous in other countries in Asia, including Thailand[25]. Based on our data, however, this postulation could not clarify the similarity in the mean age between Thai and Japanese patients with HCC, and the diversity between Thai and Taiwanese patients. Thus, it is likely that other as yet unrecognized virological factors might act as potential variables influencing the development of HCC in patients with chronic liver disease, even though they are infected with HBV of the same genotype. In addition, discrepancies regarding the role of HBV genotype might be related to variability of host and environmental factors in different geographic areas, such as genetic polym-orphism and aflatoxin exposure.
Regarding the impact of HBV genotypes on the prognosis of HCC, a prospective study in Japan demon-strates that patients with genotype C tend to have relatively poor clinical outcomes after transcatheter arterial emboli-zation (TACE) therapy compared with those with genotype B[26]. This finding is consistent with a recent report from Taiwan indicating that patients with genotype C exhibit a greater recurrent rate after curative resection of the tumor compared with those with genotype B[27]. However, our findings showed that the clinical features at presentation and overall survival of patients with HCC did not depend on the HBV genotype. The similarity of the tumor charact-eristics and clinical outcome of patients with genotypes B and C in this study was supported by the data of a recent case-control report conducted in Hong Kong[28]. Therefore, it would appear that in Thai populations, once HCC has developed, the course of the disease might be independent of the underlying HBV infection because the proportion of surviving patients is similar, irrespective of the infecting genotype. It should also be noted that overall median survival observed in this study was much shorter compared to other reports. The low survival rates in Thai patients with HCC in this study in part resulted from advanced stages of the cancer at the time of the diagnosis, while only minorities had an early detection in the course of follow-up programs.
In summary, our study demonstrated that patients with HBV genotype C, compared to those with genotype B, had a higher positive rate of HBeAg and exhibited earlier progression of cirrhosis and HCC. Genotype by itself, however, might not be responsible for an increased oncogenic effect because there was no difference in the risk of develo-ping HCC and its prognosis between patients with genotypes B and C. Further large-scale prospective studies, which offer advantages over cross-sectional investigations, are needed to establish the existence of these observations in the future.
ACKNOWLEDGMENTS
This study was supported by the Thailand Research Fund [Research Scholar (PT), and Senior Research Scholar (YP)], and Center of Excellence, Viral Hepatitis Research Unit, Chulalongkorn University.
Footnotes
Supported by the Thailand Research Fund and Center of Excellence, Viral Hepatitis Research Unit, Chulalongkorn University
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Kidd-Ljunggren K, Miyakawa Y, Kidd AH. Genetic variability in hepatitis B viruses. J Gen Virol. 2002;83:1267–1280. doi: 10.1099/0022-1317-83-6-1267. [DOI] [PubMed] [Google Scholar]
- 3.Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002;83:2059–2073. doi: 10.1099/0022-1317-83-8-2059. [DOI] [PubMed] [Google Scholar]
- 4.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554–559. doi: 10.1016/s0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 5.Lindh M, Hannoun C, Dhillon AP, Norkrans G, Horal P. Core promoter mutations and genotypes in relation to viral replication and liver damage in East Asian hepatitis B virus carriers. J Infect Dis. 1999;179:775–782. doi: 10.1086/314688. [DOI] [PubMed] [Google Scholar]
- 6.Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E, et al. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology. 2001;34:590–594. doi: 10.1053/jhep.2001.27221. [DOI] [PubMed] [Google Scholar]
- 7.Chan HL, Wong ML, Hui AY, Hung LC, Chan FK, Sung JJ. Hepatitis B virus genotype C takes a more aggressive disease course than hepatitis B virus genotype B in hepatitis B e antigen-positive patients. J Clin Microbiol. 2003;41:1277–1279. doi: 10.1128/JCM.41.3.1277-1279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao JH, Wu NH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes and the response to interferon therapy. J Hepatol. 2000;33:998–1002. doi: 10.1016/s0168-8278(00)80135-x. [DOI] [PubMed] [Google Scholar]
- 9.Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425–1430. doi: 10.1053/jhep.2002.37139. [DOI] [PubMed] [Google Scholar]
- 10.Orito E, Mizokami M, Sakugawa H, Michitaka K, Ishikawa K, Ichida T, Okanoue T, Yotsuyanagi H, Iino S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology. 2001;33:218–223. doi: 10.1053/jhep.2001.20532. [DOI] [PubMed] [Google Scholar]
- 11.Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19–26. doi: 10.1053/jhep.2003.50036. [DOI] [PubMed] [Google Scholar]
- 12.Fujie H, Moriya K, Shintani Y, Yotsuyanagi H, Iino S, Koike K. Hepatitis B virus genotypes and hepatocellular carcinoma in Japan. Gastroenterology. 2001;120:1564–1565. doi: 10.1053/gast.2001.24501. [DOI] [PubMed] [Google Scholar]
- 13.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 14.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 15.Lindh M, Gonzalez JE, Norkrans G, Horal P. Genotyping of hepatitis B virus by restriction pattern analysis of a pre-S amplicon. J Virol Methods. 1998;72:163–174. doi: 10.1016/s0166-0934(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 16.Ding X, Mizokami M, Yao G, Xu B, Orito E, Ueda R, Nakanishi M. Hepatitis B virus genotype distribution among chronic hepatitis B virus carriers in Shanghai, China. Intervirology. 2001;44:43–47. doi: 10.1159/000050029. [DOI] [PubMed] [Google Scholar]
- 17.Theamboonlers A, Jantaradsamee P, Kaew-In N, Tangkijvanich P, Hirsch P, Poovorawan Y. The predominant genotypes of hepatitis B virus in Thailand. Ann Trop Med Parasitol. 1999;93:737–743. doi: 10.1080/00034989957998. [DOI] [PubMed] [Google Scholar]
- 18.Kao JH, Chen PJ, Lai MY, Chen DS. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. J Clin Microbiol. 2002;40:1207–1209. doi: 10.1128/JCM.40.4.1207-1209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugauchi F, Chutaputti A, Orito E, Kato H, Suzuki S, Ueda R, Mizokami M. Hepatitis B virus genotypes and clinical manifestation among hepatitis B carriers in Thailand. J Gastroenterol Hepatol. 2002;17:671–676. doi: 10.1046/j.1440-1746.2002.02754.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee CM, Chen CH, Lu SN, Tung HD, Chou WJ, Wang JH, Chen TM, Hung CH, Huang CC, Chen WJ. Prevalence and clinical implications of hepatitis B virus genotypes in southern Taiwan. Scand J Gastroenterol. 2003;38:95–101. doi: 10.1080/00365520310000500. [DOI] [PubMed] [Google Scholar]
- 21.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 22.Yuen MF, Sablon E, Yuan HJ, Wong DK, Hui CK, Wong BC, Chan AO, Lai CL. Significance of hepatitis B genotype in acute exacerbation, HBeAg seroconversion, cirrhosis-related complications, and hepatocellular carcinoma. Hepatology. 2003;37:562–567. doi: 10.1053/jhep.2003.50098. [DOI] [PubMed] [Google Scholar]
- 23.Orito E, Mizokami M. Hepatitis B virus genotypes and hepatocellular carcinoma in Japan. Intervirology. 2003;46:408–412. doi: 10.1159/000075000. [DOI] [PubMed] [Google Scholar]
- 24.Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Ueda R, et al. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J Virol. 2002;76:5985–5992. doi: 10.1128/JVI.76.12.5985-5992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Gish RG, et al. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology. 2003;124:925–932. doi: 10.1053/gast.2003.50140. [DOI] [PubMed] [Google Scholar]
- 26.Tsubota A, Arase Y, Ren F, Tanaka H, Ikeda K, Kumada H. Genotype may correlate with liver carcinogenesis and tumor characteristics in cirrhotic patients infected with hepatitis B virus subtype adw. J Med Virol. 2001;65:257–265. doi: 10.1002/jmv.2028. [DOI] [PubMed] [Google Scholar]
- 27.Chen JD, Liu CJ, Lee PH, Chen PJ, Lai MY, Kao JH, Chen DS. Hepatitis B genotypes correlate with tumor recurrence after curative resection of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2004;2:64–71. doi: 10.1016/s1542-3565(03)00293-3. [DOI] [PubMed] [Google Scholar]
- 28.Yuen MF, Tanaka Y, Mizokami M, Yuen JC, Wong DK, Yuan HJ, Sum SM, Chan AO, Wong BC, Lai CL. Role of hepatitis B virus genotypes Ba and C, core promoter and precore mutations on hepatocellular carcinoma: a case control study. Carcinogenesis. 2004;25:1593–1598. doi: 10.1093/carcin/bgh172. [DOI] [PubMed] [Google Scholar]


