Abstract
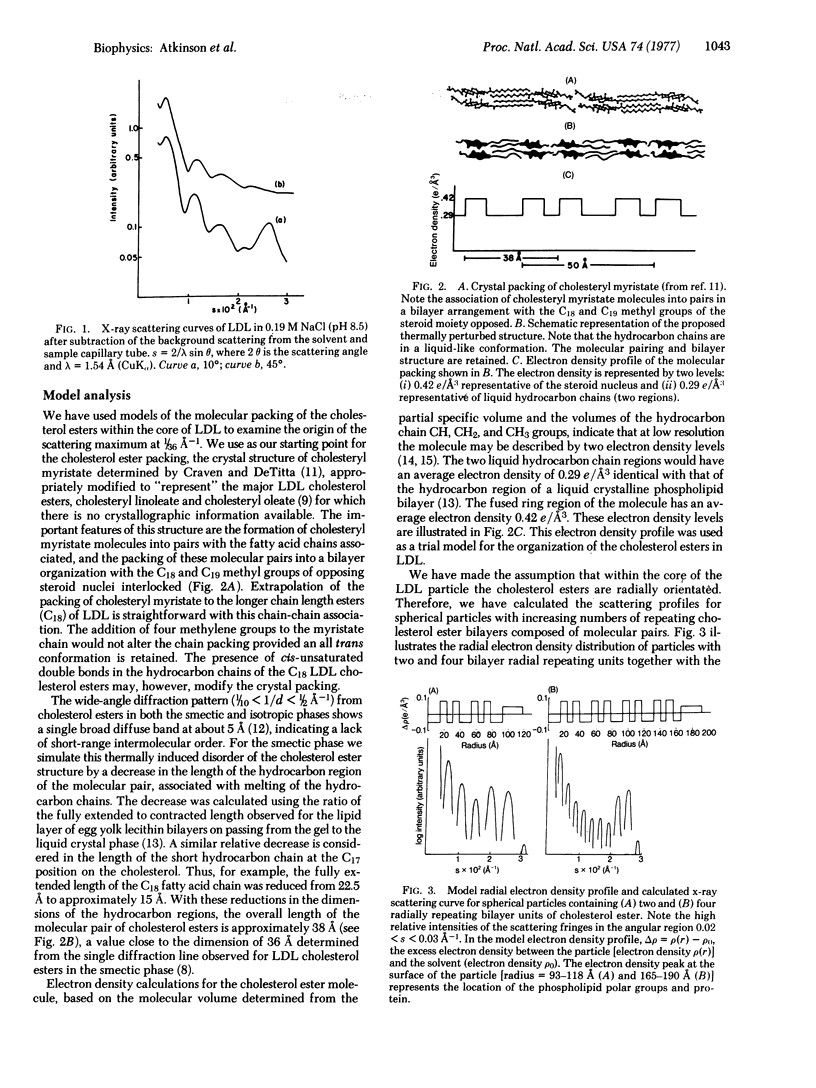
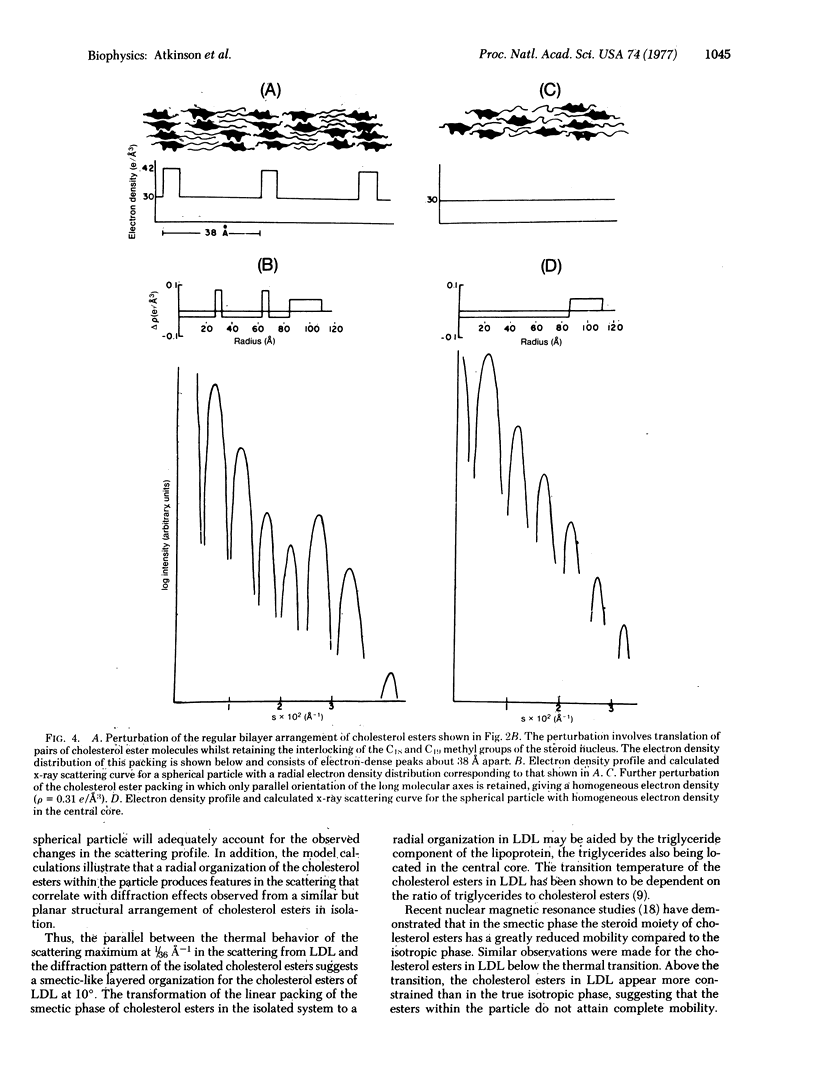
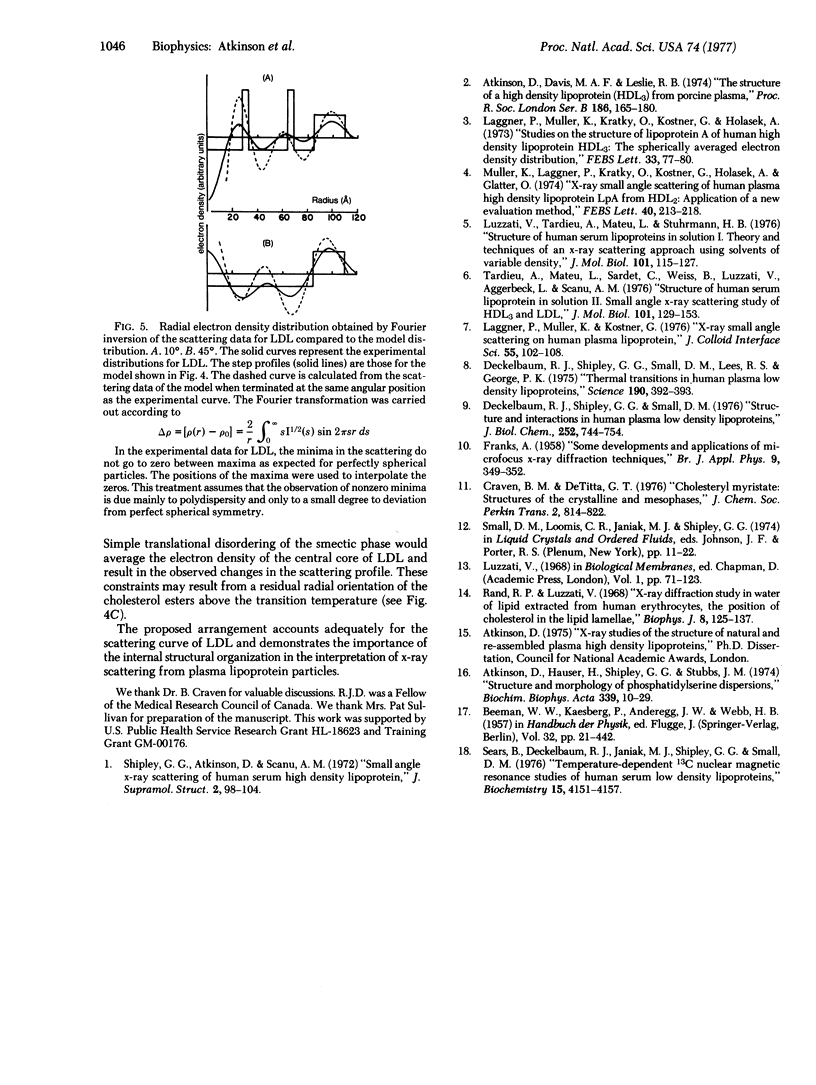
Human plasma low density lipoprotein (LDL) exhibits a thermal transition over the temperature range 20-40 degrees. This transition is associated with a structural change within the lipoprotein particle and is reflected in the small-angle x-ray scattering profiles from LDL. The scattering profile of the quasispherical LDL particle at 10 degrees shows a relatively intense maximum at 1/36 A-1 which is absent from the scattering of LDL at 45 degrees. Theoretical calculations, using model electron density distributions, have been carried out to describe the packing of arrangement of the cholesterol esters, based on perturbations of the molecular packing of crystalline cholesteryl myristate, adequately reproduces the high relative intensity of the x-ray scattering maximum at 1/36 A-1. The perturbations of the packing in the crystal structure of cholesteryl myristate involve "melting" of the hydrocarbon chains of the esters together with translations of pairs of molecules parallel to the molecular long axis. The interaction of opposing steroid moieties, with C18 and C19 angular methyl groups interlocked, exhibited in the crystal structure is retained in the perturbed arrangement. At 45 degrees, thermally induced disorder of this arrangement averages the electron density of the central core. The x-ray scattering profiles of particles with a homogeneous electron density in the core region do not show a high relative intensity of the subsidiary maxima in the 1/36 A-1 region, in agreement with experimental observation. The results of these calculations support the concept that the thermal transition observed for LDL is due to a smectic leads to disordered transition of the cholesterol esters in the core of the LDL particle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D., Davis M. A., Leslie R. B. The structure of a high density lipoprotein (HDL3) from porcine plasma. Proc R Soc Lond B Biol Sci. 1974 Jun 11;186(1083):165–180. doi: 10.1098/rspb.1974.0044. [DOI] [PubMed] [Google Scholar]
- Atkinson D., Hauser H., Shipley G. G., Stubbs J. M. Structure and morphology of phosphatidylserine dispersions. Biochim Biophys Acta. 1974 Feb 26;339(1):10–29. doi: 10.1016/0005-2736(74)90329-0. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M., Lees R. S., George P. K. Thermal transitions in human plasma low density lipoproteins. Science. 1975 Oct 24;190(4212):392–394. doi: 10.1126/science.170681. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Shipley G. G., Small D. M. Structure and interactions of lipids in human plasma low density lipoproteins. J Biol Chem. 1977 Jan 25;252(2):744–754. [PubMed] [Google Scholar]
- Laggner P., Müller K., Kratky O., Kostner G., Holasek A. Studies on the structure of lipoprotein A of human high density lipoprotein HDL3: the spherically averaged electron density distribution. FEBS Lett. 1973 Jun 15;33(1):77–80. doi: 10.1016/0014-5793(73)80163-2. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Tardieu A., Mateu L. Structure of human serum lipoproteins in solution. I. Theory and techniques of an x-ray scattering approach using solvents of variable density. J Mol Biol. 1976 Feb 25;101(2):115–127. doi: 10.1016/0022-2836(76)90367-3. [DOI] [PubMed] [Google Scholar]
- Müller K., Laggner P., Kratky O., Kostner G., Holasek A., Glatter O. X-ray small angle scattering of human plasma high density lipoprotein LpA from HDL2: application of a new evaluation method. FEBS Lett. 1974 Mar 15;40(1):213–218. doi: 10.1016/0014-5793(74)80931-2. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Luzzati V. X-ray diffraction study in water of lipids extracted from human erythrocytes: the position of cholesterol in the lipid lamellae. Biophys J. 1968 Jan;8(1):125–137. doi: 10.1016/S0006-3495(68)86479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears B., Deckelbaum R. J., Janiak M. J., Shipley G. G., Small D. M. Temperature-dependent 13C nuclear magnetic resonance studies of human serum low density lipoproteins. Biochemistry. 1976 Sep 21;15(19):4151–4157. doi: 10.1021/bi00664a003. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Atkinson D., Scanu A. M. Small-angle x-ray scattering of human serum high-density lipoproteins. J Supramol Struct. 1972;1(2):98–104. doi: 10.1002/jss.400010203. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Mateu L., Sardet C., Weiss B., Luzzati V. Structure of human serum lipoproteins in solution. II. Small-angle x-ray scattering study of HDL and LDL. J Mol Biol. 1976 Feb 25;101(2):129–153. doi: 10.1016/0022-2836(76)90368-5. [DOI] [PubMed] [Google Scholar]



