Abstract
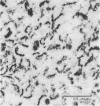
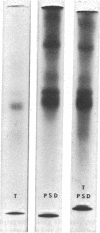
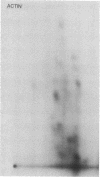
The postsynaptic density is a unique subcellular organelle associated with the synaptic complex and appears as an electron-dense area immediately subjacent to the postsynaptic plasma membrane. The postsynaptic density was isolated from the synaptosomal fraction and the protein constituents were analyzed by polyacrylamide gel electrophoresis. Polypeptides closely related to tubulin were identified as a major component of the postsynaptic density on the basis of molecular weight, subunit structure, and peptide map criteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blitz A. L., Fine R. E. Muscle-like contractile proteins and tubulin in synaptosomes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4472–4476. doi: 10.1073/pnas.71.11.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Brownlee S. M. Peptide mapping of proteins from acrylamide gels. Anal Biochem. 1973 Sep;55(1):213–221. doi: 10.1016/0003-2697(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Levy W., Taylor D. An ultrastructural and chemical analysis of the effect of triton X-100 on synaptic plasma membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):406–418. doi: 10.1016/0005-2736(71)90119-2. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972 Dec;55(3):696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Witman G. B., Rosenbaum J. L. Directionality of brain microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1710–1714. doi: 10.1073/pnas.71.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A. Properties of rat brain tubulin. J Biol Chem. 1974 Mar 10;249(5):1407–1416. [PubMed] [Google Scholar]
- Feit H., Barondes S. H. Colchicine-binding activity in particulate fractions of mouse brain. J Neurochem. 1970 Sep;17(9):1355–1364. doi: 10.1111/j.1471-4159.1970.tb06870.x. [DOI] [PubMed] [Google Scholar]
- Feit H., Dutton G. R., Barondes S. H., Shelanski M. L. Microtubule protein. Identification in and transport to nerve endings. J Cell Biol. 1971 Oct;51(1):138–147. doi: 10.1083/jcb.51.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit H., Slusarek L., Shelanski M. L. Heterogeneity of tubulin subunits. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2028–2031. doi: 10.1073/pnas.68.9.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E. G. Synaptic fine structure and nuclear, cytoplasmic and extracellular networks: The stereoframework concept. J Neurocytol. 1975 Jun;4(3):315–339. doi: 10.1007/BF01102116. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Identification of glycoproteins and proteins at synapses in the central nervous system. J Biol Chem. 1977 Jan 25;252(2):786–793. [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Intermolecular disulfide bonds at central nervous system synaptic junctions. Biochem Biophys Res Commun. 1976 Dec 20;73(4):858–864. doi: 10.1016/0006-291x(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Luttges M. W. Electrophoretic separation of nervous system proteins on exponential gradient polyacrylamide gels. J Neurochem. 1975 May;24(5):1077–1079. doi: 10.1111/j.1471-4159.1975.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metuzals J., Mushynski W. E. Electron microscope and experimental investigations of the neurofilamentous network in Deiters' neurons. Relationship with the cell surface and nuclear pores. J Cell Biol. 1974 Jun;61(3):701–722. doi: 10.1083/jcb.61.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B., Witman G. B., Carlson K., Rosenbaum J. L. Comparison of the microtubule proteins of neuroblastoma cells, brain, and Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2273–2277. doi: 10.1073/pnas.68.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politoff A. L., Rose S., Pappas G. D. The calcium binding sites of synaptic vesicles of the frog sartorius neuromuscular junction. J Cell Biol. 1974 Jun;61(3):818–823. doi: 10.1083/jcb.61.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters B. B., Matus A. I. Tubulin in postynaptic junctional lattice. Nature. 1975 Oct 9;257(5526):496–498. doi: 10.1038/257496a0. [DOI] [PubMed] [Google Scholar]
- Yen S. H., Dahl D., Schachner M., Shelanski M. L. Biochemistry of the filaments of brain. Proc Natl Acad Sci U S A. 1976 Feb;73(2):529–533. doi: 10.1073/pnas.73.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]