Abstract
AIM: The aims of this study were to explore individualized treatment method for hepatocellular carcinoma (HCC) patients whose maximum tumor size was less than 5 cm to improve prognosis and survival quality.
METHODS: Thirty cases of primary HCC patients undergoing tumor resection were retrospectively analyzed (resection group). All the tumors were proved as primary HCC with pathologic examination. The patients were divided into two groups according to follow-up results: group A, with tumor recurrence within 1 year after resection; group B, without tumor recurrence within 1 year. Immunohist-ochemical stainings were performed using 11 kinds of monoclonal antibodies (AFP, c-erbB2, c-met, c-myc, HBsAg, HCV, Ki-67, MMP-2, nm23-H1, P53, and VEGF), and expressing intensities were quantitatively analyzed. Regression equation using factors affecting prognosis of HCC was constructed with binary logistic method. HCC patients undergoing percutaneous microwave coagulation therapy (PMCT) were also retrospectively analyzed (PMCT group). Immunohistochemical stainings of tumor biopsy samples were performed with molecules related to HCC prognosis, staining intensities were quantitatively analyzed, coincidence rate of prediction was calculated.
RESULTS: In resection group, the expressing intensities of c-myc, Ki-67, MMP-2 and VEGF in cancer tissue in group A were significantly higher than those in group B (t = 2.97, P = 0.01; t = 2.42, P = 0.03<0.05; t = 2.57, P = 0.02<0.05; t = 3.43, P = 0.004<0.01, respectively); the expressing intensities of 11 kinds of detected molecules in para-cancer tissue in groups A and B were not significantly different (P>0.05). The regression equation predicting prognosis of HCC is as follows: P(1) = 1/[1+e-(3.663-0.412mycc-2.187Ki-67c-0.397vegfc)]. It demonstrates that prognosis of HCC in resection group was related with c-myc, Ki-67 and VEGF expressing intensity in cancer tissue. In PMCT group, the expressing intensities of c-myc, Ki-67 and VEGF in cancer tissue in group A were significantly higher than those in group B (t = 4.57, P = 0.000<0.01; t = 2.08, P = 0.04<0.05; t = 2.38, P = 0.02<0.05, respectively); the expressing intensities of c-myc, Ki-67 and VEGF in para-cancer tissue in groups A and B were not significantly different (P>0.05). The coincidence rate of patients undergoing PMCT in group A was 88.00% (22/25), in group B 68.75% (11/16), the total coincidence rate was 80.49% (33/41).
CONCLUSION: The regression equation is accurate and feasible and could be used for predicting prognosis of HCC, it helps to select treatment method (resection or PMCT) for HCC patients to realize individualized treatment to improve prognosis.
Keywords: Hepatocellular carcinoma, Prognosis, Prediction
INTRODUCTION
Originally resection was the first choice for hepatocellular carcinoma (HCC), however, high recurrence rate after resection was a major problem influencing the therapeutic effect[1]. Percutaneous microwave coagulation therapy (PMCT) plays an important role in the treatment for HCC. Some patients survived for more than 5 years after PMCT. PMCT has the superiority of enhancing immune function both in the tumor and the whole body. An increased systemic immune response directed against the tumor may play an important role in improved survival for HCC patients[2].
Individualized therapy is one new trend in cancer treatment in the 21st century. The purpose of individualized treatment is to improve prognosis, as appropriate therapy method is selected for each patient[3]. There are no sufficient theoretical fundamentals for HCC patients to either select resection or PMCT for therapy. Recurrence and/or metastasis are the main factors affecting prognosis of HCC[4,5]. Patients’ clinical conditions before resection and therapeutic measures after operation may be similar, however, their prognosis after resection may differ largely. Some have tumor recurrence within 1 year after resection, while others do not, and the reason leading to this difference is not clear. The occurrence and prognosis of HCC is related to activation of proto-oncogene, inactivation of tumor suppressor gene, abnormal expression of growth factors and/or their receptors[6-8].
The way to realize individualized treatment is to predict prognosis of HCC patients before treatment, and then according to the prediction suitable treatment methods are selected to best improve prognosis. Therefore, how to predict prognosis of HCC patients becomes the major problem. The aims of this study were: (1) to compare the expressing intensity of 11 kinds of molecules related to HCC in resection group; (2) to construct a regression equation predicting prognosis of HCC patients; (3) to verify the accuracy of the regression equation in PMCT group.
MATERIALS AND METHODS
Patients
Thirty cases of primary HCC patients undergoing resection (resection group) in the Department of Hepatobiliary Surgery in General Hospital of PLA of China (301 Hospital) were retrospectively analyzed. The selecting standards listed were as follows: solitary nodule, maximum size less than 5 cm, no transarterial chemoembolization (TACE) or local thermal therapy performed before resection, no other specific treatments performed after resection. All the tumors were proved as primary HCC with pathologic examination. The patients were divided into two groups according to follow-up results: group A, with tumor recurrence within 1 year after resection; group B, without tumor recurrence within 1 year. The differences of clinical data were not significant (P>0.05, Table 1).
Table 1.
Clinical data of patients in resection group.
| Item | Group A (n = 15) | Group B (n = 15) |
| Sex (m/f) | 14 /1 | 14 /1 |
| Age (yr) | 54.7±14.3 | 48.2±8.4 |
| Mean diameter (cm) | 3.2±1.0 | 2.9±1.1 |
| Child-pugh grade | A | A |
| Serum AFP (mg/L) | 180±200 | 120±200 |
| ALT (U/L) | 58.0±54.1 | 64.9±56.6 |
| AST (U/L) | 49.9±46.8 | 42.0±37.7 |
| HBsAg positive rate | 87% (13/15) | 100 (15/15) |
Forty-one cases of primary HCC patients undergoing PMCT (PMCT group) in the Department of Ultrasound in General Hospital of PLA of China (301 Hospital) were retrospectively analyzed. The selecting standards were the same as those in resection group. It included 36 males and 5 females. All the nodules were biopsied under ultrasonic guidance and proved as primary HCC with pathologic examination. The patients were divided into two groups according to follow-up results: group A, with tumor recurrence within 1 year after PMCT; group B, without tumor recurrence within 1 year. The differences of clinical data were not significant (P>0.05, Table 2).
Table 2.
Clinical data of patients in PMCT group.
| Item | Group A (n = 25) | Group B (n = 16) |
| Sex (m/f) | 21/4 | 15/1 |
| Age (yr) | 55.8±13.4 | 57.2±11.2 |
| Mean diameter (cm) | 3.5±1.0 | 3.0±0.8 |
| Child-pugh grade | A | A |
| Serum AFP (mg/L) | 160±200 | 120±160 |
| ALT (U/L) | 52.0±42.5 | 48.1±40.1 |
| AST (U/L) | 51.9±38.9 | 41.2±36.5 |
| HBsAg positive rate | 76% (16/21) | 95 (20/21) |
Immunohistochemical staining
HPIAS-1000 Diagram-Writing Analyzing System was produced by Wuhan Champion Image Technology Corporation Ltd. SP and DAB kit, monoclonal antibodies of AFP, c-erbB2, c-met, c-myc, HBsAg, HCV, Ki-67, MMP-2, nm23-H1, P53 and VEGF were all purchased from Beijing Zhongshan Biological Technology Corporation Ltd. Serial sections were made with wax sample of resected tumors, the thickness of the slice was 4 μm. Immunohistochemical staining was performed with SP three-step method using the monoclonal antibodies listed above.
Quantitative analysis of detected molecules
Quantitative analysis of the detected molecules was performed with HPIAS-1000 imaging analysis system. The molecules in cancer and para-cancer tissues were both analyzed. Three fields of view (FOVs) were randomly selected in cancer tissue and para-cancer tissue to quantitatively analyze the expressing intensity. One hundred cells were observed in each FOV, positive-staining cells were calculated, finally the average positive-staining cells in 100 observed cells were determined. The cells were determined as positive-staining cells only if they were stained, without considering their staining intensity. The medium optical density (MOD) of plasm or nucleus in positive-staining cells was calculated, the product of positive-staining cells and MOD was calculated, which was considered as expressing the intensity of positive-staining molecules[9].
Statistical analysis
Data were presented as mean±SD. Paired-sample t test was used to compare the difference, the statistic software SPSS 10.0 was used. Recurrence state after treatment (resection or PMCT) was defined as 0 or 1: that with tumor recurrence within 1 year after treatment was defined as 0 (group A), that without tumor recurrence within 1 year was defined as 1 (group B). Regression equation predicting prognosis of HCC was constructed using binary logistic regression analysis[10]. In this study, P<0.05 was considered statistically significant.
RESULTS
Expressing intensity of detected molecules in resection group
The expressing intensities of c-myc, Ki-67, MMP-2, and VEGF in cancer tissue in group A were significantly higher than those in group B (Table 3).
Table 3.
Expressing intensity of detected molecules in cancer tissue in groups A and B.
| Molecule | Group A | Group B |
| AFP | 2.72±2.93 | 1.55±3.00 |
| c-erbB-2 | 9.44±5.02 | 6.82±6.10 |
| c-met | 2.04±2.68 | 1.61±3.22 |
| c-myc | 3.95±2.81b | 1.34±2.74 |
| HBsAg | 0.45±0.96 | 2.26±5.04 |
| HCV | 0.51±1.40 | 0.96±2.44 |
| Ki-67 | 1.57±2.20a | 0.18±0.38 |
| MMP-2 | 3.70±4.13a | 0.61±1.70 |
| Nm23-H1 | 2.18±3.05 | 1.49±2.20 |
| P53 | 1.31±3.37 | 0.39±1.16 |
| VEGF | 5.44±4.20b | 1.04±3.38 |
P<0.05,
P<0.01 vs group B.
The expressing intensities of 11 kinds of detected molecules in para-cancer tissue in groups A and B were not significantly different (Table 4).
Table 4.
Expressing intensity of 11 detected molecules in para-cancer tissue in groups A and B.
| Molecule | Group A | Group B |
| AFP | 0.09±0.35 | 0.00±0.00 |
| c-erbB-2 | 0.76±2.50 | 0.01±0.04 |
| c-met | 0.00±0.00 | 0.00±0.00 |
| c-myc | 0.00±0.00 | 0.26±1.00 |
| HbsAg | 9.02±5.83 | 9.11±6.19 |
| HCV | 1.32±2.88 | 0.08±0.20 |
| Ki-67 | 0.57±2.22 | 0.01±0.04 |
| MMP-2 | 0.80±3.10 | 0.00±0.00 |
| Nm23-H1 | 0.33±0.81 | 0.00±0.00 |
| P53 | 0.00±0.00 | 0.00±0.00 |
| VEGF | 0.04±0.15 | 0.00±0.00 |
Micrographs of c-myc, Ki-67, MMP-2 and VEGF
The micrographs of c-myc, Ki-67, MMP-2, and VEGF expression are shown in Figures 1, 2, 3, 4.
Figure 1.
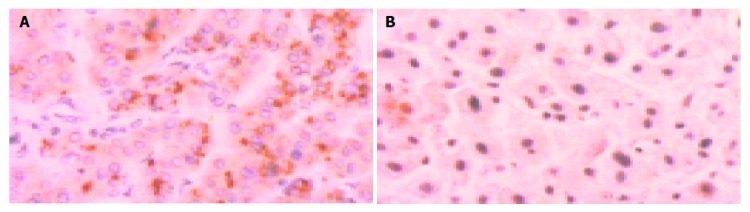
Positive and negative c-myc (×400). A: Positive c-myc; B: Negative c-myc.
Figure 2.
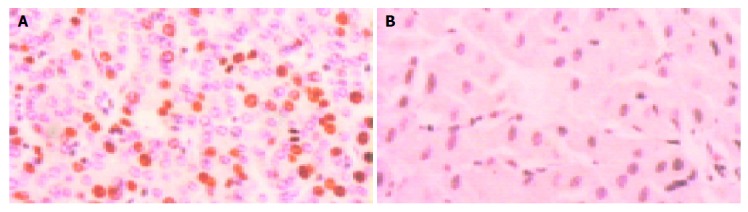
Positive and negative Ki-67 (×400). A: Positive Ki-67; B: Negative Ki-67.
Figure 3.
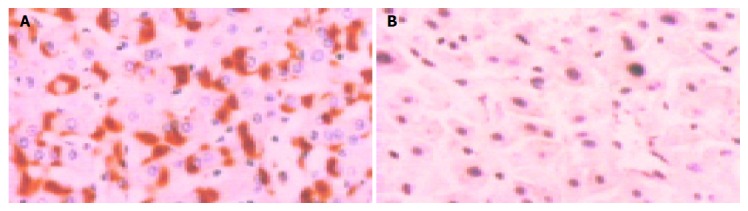
Positive and negative MMP-2 (×400). A: Positive MMP-2; B: Negative MMP-2.
Figure 4.
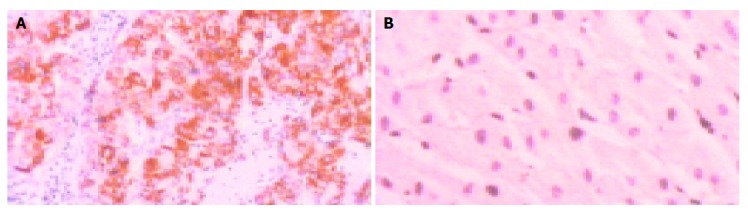
Positive and negative VEGF (×400). A: Positive VEGF; B: Negative VEGF.
Construction of regression equation
From the tables listed above, it demonstrated that c-myc, Ki-67, MMP-2, and VEGF were perhaps key factors determining prognosis difference of patients in groups A and B. Recurrence state (0 or 1) was used as a dependent variable; expressing intensities of c-myc, Ki-67, MMP-2, and VEGF in cancer tissue in groups A and B were used as covariates to construct an equation with binary logistic regression analysis.
The regression equation predicting prognosis of HCC is as follow:
P(1) = 1/[1+e–(3.663–0.412mycc-2.187Ki-67c-0.397vegfc)]
The variables in the regression equation are listed in Table 5.
Table 5.
Variables in the regression equation.
| B | SE | Wald | df | Sig | Exp (B) |
95.0% CI for EXP (B) |
|||
| Lower | Upper | ||||||||
| Step 1 | MYCC | –0.357 | 0.213 | 2.807 | 1 | 0.094 | 0.700 | 0.461 | 1.063 |
| KI-67C | –2.118 | 1.251 | 2.865 | 1 | 0.091 | 0.120 | 0.010 | 1.397 | |
| MMP-2C | –0.244 | 0.306 | 0.638 | 1 | 0.425 | 0.783 | 0.430 | 1.426 | |
| VEGFC | –0.367 | 0.160 | 5.269 | 1 | 0.022 | 0.693 | 0.506 | 0.948 | |
| Constant | 3.680 | 1.363 | 7.289 | 1 | 0.007 | 39.64 | |||
| Step 2 | MYCC | –0.412 | 0.211 | 3.795 | 1 | 0.051 | 0.663 | 0.438 | 1.003 |
| KI-67C | –2.187 | 1.271 | 2.959 | 1 | 0.085 | 0.112 | 0.009 | 1.356 | |
| VEGFC | –0.397 | 0.159 | 6.222 | 1 | 0.013 | 0.672 | 0.492 | 0.918 | |
| Constant | 3.663 | 1.410 | 6.750 | 1 | 0.009 | 38.991 | |||
Variable(s) entered on step 1: MYCC, KI-67C, MMP-2C, VEGFC.
MYCC: c-myc expressing intensity in cancer tissue; Ki-67c: ki-67 expressing intensity in cancer tissue; VEGFC: vegf expressing intensity in cancer tissue.
Expressing intensity of detected molecules in PMCT group
The expressing intensities of c-myc, Ki-67, and VEGF in cancer tissue in group A were significantly higher than those in group B (Table 6).
Table 6.
Expressing intensity of detected molecules in cancer tissue in groups A and B.
| Molecule | Group A | Group B |
| c-myc | 8.31±4.71b | 2.52±2.30 |
| Ki-67 | 1.53±1.79a | 0.47±1.19 |
| VEGF | 6.23±5.44a | 2.88±3.57 |
P<0.05,
P<0.01 vs group B.
The expressing intensities of c-myc, Ki-67, and VEGF in para-cancer tissue in groups A and B were not significantly different (Table 7).
Table 7.
Expressing intensity of detected molecules in para-cancer tissue in groups A and B.
| Molecule | Group A | Group B |
| c-myc | 2.20±3.91 | 0.95±1.84 |
| Ki-67 | 6.40×103±3.20×102 | 0.00±0.00 |
| VEGF | 0.66±1.84 | 0.54±2.16 |
Verification of regression equation
The values of expressing intensities of c-myc, Ki-67, and VEGF in PMCT group were introduced into the regression equation, coincidence rate of prediction was calculated. Coincidence rate in group A was 88.00% (22/25) that in group B was 68.75% (11/16); total coincidence rate was 80.49%.
DISCUSSION
The expressing intensities of AFP, c-erbB2, c-met, c-myc, MMP-2, nm23-H1, VEGF in cancer tissue were higher than those in para-cancer tissue in group A. The expressing intensities of c-erbB2 and nm23-H1 in cancer tissue were higher than those in para-cancer tissue in group B. The expressing intensity of HBsAg in para-cancer tissue was higher than that in cancer tissue in groups A and B. The expressing intensities of c-myc, Ki-67, MMP-2 and VEGF in cancer tissue in group A were higher than those in group B, the expressing intensities of the 11 kinds of molecules listed in this study in para-cancer tissue in groups A and B were not significantly different.
It was reported in the literature that AFP[11,12], c-erbB-2[13], c-met[14], HBsAg[15], HCV[16], nm23-H1 and P53[17] expressions were related to prognosis of HCC, however, it demonstrated in this study that their expressions were not significantly related to prognosis of HCC in resection group. The reason was probably that the clinical characters of patients in groups A and B were similar. These seven kinds of molecules played minor roles in determining malignancy of HCC in this study. The expressing intensities of c-myc, Ki-67, MMP-2, and VEGF in groups A and B in resection group were significantly different. The clinical data in groups A and B were similar, there might be some key molecules closely related to prognosis difference of groups A and B, and these four kinds of molecules were perhaps such key molecules.
Matrix metalloproteinases (MMPs) specially degrade extracellular matrix (ECM) and basement membranes, they are involved in tissue remodeling and angiogenesis of HCC. Ishii et al[18], reported that MMP-2 was associated with carcinogenesis and progression of HCC. Kuyvenhoven et al, measured serum MMP-2 by ELISA in 91 patients with chronic liver disease, including 25 patients with hepatocellular carcinoma (HCC), and in 60 controls, they demonstrated that MMP-2 was significantly higher in patients with chronic liver disease compared to controls, and increased with Child-Pugh class. There was a significant correlation between MMP-2 and liver function (bilirubin, albumin, and prothrombin time). MMP-2 levels in patients with HCC were significantly higher than those in controls. Serum MMP-2 correlates with the severity of liver disease and may reflect changes in extracellular matrix remodeling[19]. It was demonstrated in this study that the expressing intensity of MMP-2 in cancer tissue was much higher than that in para-cancer tissue in group A, and it was much higher in cancer tissue in group A than that in group B. It seems that MMP-2 was related to prognosis of HCC.
Over-expression of c-myc oncogene could promote cellular proliferation and cancerous invasion, it is related to prognosis of HCC. Wang et al, evaluated the association of c-myc amplification with the prognosis of patients with HCC, they demonstrated that c-myc amplifications in single nodular and multiple-nodular HCCs were significantly different (12% vs 38%, P<0.01). More frequent c-myc amplification was detected in metastatic HCC (45%) compared with primary HCC (29%) and in recurrent HCC (60%) compared with primary HCC (38%). The results strongly suggest that amplification of the c-myc oncogene was correlated with a poor prognosis[20]. It was demonstrated in vitro that the block of c-myc expression could suppress the growth of hepatoma cells[21].
Ki-67 proliferation associated antigen was used for determining the cellular proliferation and prognosis of patients with tumor. Ki-67 could label any proliferating cells in cellular cycle except cells in G0 stage, however, it could not label cells in silent period, so Ki-67 was considered as an objective marker indicating cell proliferation. Ki-67 locates in cell nucleus, it is always in particle shapes in immunohistochemical staining. King et al, reported 67 cases of HCC undergoing tumor resection, no other treatment (TAE, TACE) was performed before resection. Immunohistochemical staining showed that Ki-67 labeling index in cancer tissue was much higher than that in normal liver, the survival period of HCC patients with a Ki-67 labeling index >10% was shorter than those with a Ki-67 labeling index ≤10%. Ki-67 expression helped to select treatment method after tumor resection[22]. Ito et al[23], reported that HCCs with high expression of Ki-67 had the characters of poor cell differentiation, with portal vein invasion, high chance of stage III and intrahepatic metastasis, short survival period.
Vascular endothelial growth factor (VEGF) is known to promote the development of new blood vessels, which is fundamental to tumor growth and metastasis. Expression of VEGF in HCC tissue was positively correlated with growth and metastasis of HCC, it could be a marker for determining prognosis of HCC[24,25]. Expression of VEGF was strongly correlated with microvessel density (MVD) and tumor size[26,27]. Overproduction of the angiogenic growth factor VEGF by HCC cells may increase vascularity and tumor growth in a paracrine manner[28]. Preoperative serum VEGF was increased in patients with resectable HCC compared with healthy controls. Increased serum VEGF was correlated with HCC recurrence[29]. Multivariate analysis showed that serum VEGF was the most significant predictor of disease-free survival (DFS) and overall survival (OS) in HCC patients after surgical resection. So preoperative serum VEGF is a significant independent predictor of tumor recurrence, DFS, and OS in patients with resectable HCC[30]. The frequency of venous invasion in HCC patients with a high serum VEGF level was significantly greater compared with patients with a low serum VEGF level. It demonstrated that a high preoperative serum VEGF level was a predictor of microscopic venous invasion in HCCs and could be used as a biologic marker of tumor invasiveness and a prognostic factor in HCCs[31,32]. The expression of VEGF mRNA was higher in HCCs with portal vein tumor thrombus (PVTT) than that without PVTT. PVTT was more often seen in HCC patients with positive expression of VEGF mRNA than in patients who had negative expression. VEGF correlated well with the formation of PVTT of HCC[33].
A regression equation predicting prognosis of HCC using binary logistic regression analysis was constructed, it demonstrated in the equation that c-myc, Ki-67, and VEGF expressions were greatly related to prognosis of HCC, however, MMP-2 did not enter the equation, which demonstrated that MMP-2 was not closely related to prognosis of HCC. Taken together, it could be inferred that high expressions of c-myc, Ki-67, and VEGF in HCC tissue were related to prognosis of HCC. The regression equation was verified further with patients in PMCT group, it demonstrated that total coincidence rate was 80.49%, it could be used as a kind of prognosis predicting method for HCC patients, and help HCC patients to select individualized treatment method.
Some scholars predicted that the focal point of HCC research in the 21st century would be recurrence and/or metastasis. With currently available techniques (surgical resection or thermal coagulation), it is no problem to have HCC nodules with maximum size less than 5 cm fully resected or coagulated. Post-treatment recurrence is a problem puzzling clinicians. Some recurrence occurs within 1 year after resection. Considering that surgical resection for HCC had the disadvantage of suppressing immunological function at an early stage, PMCT treatment for HCC had the advantage of enhancing local tumor and whole-body immunological functions[2]. The following points should be considered in choosing treatment methods for HCC whose maximum size is less than 5 cm: ultrasound guided biopsy should be performed for suspected HCC patients to confirm its pathological diagnosis; if it is diagnosed as HCC, immunohistochemical staining of c-myc, Ki-67, and VEGF and quantitative analysis of the molecules should be performed; the prognosis of the patients could be predicted with the regression equation in this paper. If the predicted value is less than 0.5, then the patient has the tendency of high recurrence even after surgical resection; if the predicted value is more than 0.5, then the patient does not have the tendency of high recurrence after surgical resection. The former, micro-trauma methods such as PMCT or TACE should be chosen for treatment, meanwhile, other auxiliary methods such as biological and traditional Chinese medicine treatment should be adopted, and moreover, close follow-up should be done to detect new lesions. For the latter, surgical resection is suggested.
Footnotes
Supported by the Medical and Health Science Foundation of PLA During the 10th five-year plan period, No. 01Z038
Science Editor Zhu LH Language Editor Elsevier HK
References
- 1.Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36–41. doi: 10.1002/jso.10284. [DOI] [PubMed] [Google Scholar]
- 2.Dong BW, Zhang J, Liang P, Yu XL, Su L, Yu DJ, Ji XL, Yu G. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperthermia. 2003;19:119–133. doi: 10.1080/0265673021000017154. [DOI] [PubMed] [Google Scholar]
- 3.Levy AE, Kowdley KV. Unresectable hepatocellular carcinoma: the need for an individualized multidisciplinary approach. J Clin Gastroenterol. 2001;33:180–182. doi: 10.1097/00004836-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Harrison LE, Koneru B, Baramipour P, Fisher A, Barone A, Wilson D, Dela Torre A, Cho KC, Contractor D, Korogodsky M. Locoregional recurrences are frequent after radiofrequency ablation for hepatocellular carcinoma. J Am Coll Surg. 2003;197:759–764. doi: 10.1016/S1072-7515(03)00750-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen JY, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Clinicopathologic features and factors related to survival of patients with small hepatocellular carcinoma after hepatic resection. World J Surg. 2003;27:294–298. doi: 10.1007/s00268-002-6539-6. [DOI] [PubMed] [Google Scholar]
- 6.Daveau M, Scotte M, François A, Coulouarn C, Ros G, Tallet Y, Hiron M, Hellot MF, Salier JP. Hepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinoma. Mol Carcinog. 2003;36:130–141. doi: 10.1002/mc.10103. [DOI] [PubMed] [Google Scholar]
- 7.Armengol C, Boix L, Bachs O, Solé M, Fuster J, Sala M, Llovet JM, Rodés J, Bruix J. p27(Kip1) is an independent predictor of recurrence after surgical resection in patients with small hepatocellular carcinoma. J Hepatol. 2003;38:591–597. doi: 10.1016/s0168-8278(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Sugawara Y, Shi YZ, Makuuchi M. Telomerase expression and p53 status in hepatocellular carcinoma. Am J Gastroenterol. 2002;97:3166–3171. doi: 10.1111/j.1572-0241.2002.07125.x. [DOI] [PubMed] [Google Scholar]
- 9.Ling X, Chen W, Liu S, Wang G. Expression of TGF-beta in region of bone defect repaired by collagen/nano-beta-tricalcium phosphate composite artificial bone. J Huazhong Univ Sci Technolog Med Sci. 2003;23:302–305. doi: 10.1007/BF02829522. [DOI] [PubMed] [Google Scholar]
- 10.Baech J, Roer O, Johnsen HE. Individual quality assessment of autografting by probability evaluation: a model estimated by analysis of graft-related end points in 204 patients with malignancies. Bone Marrow Transplant. 2003;31:453–458. doi: 10.1038/sj.bmt.1703828. [DOI] [PubMed] [Google Scholar]
- 11.Tan CK, Law NM, Ng HS, Machin D. Simple clinical prognostic model for hepatocellular carcinoma in developing countries and its validation. J Clin Oncol. 2003;21:2294–2298. doi: 10.1200/JCO.2003.03.151. [DOI] [PubMed] [Google Scholar]
- 12.Ueno S, Tanabe G, Nuruki K, Oketani M, Komorizono Y, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Prognosis of hepatocellular carcinoma associated with Child class B and C cirrhosis in relation to treatment: a multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg. 2002;9:469–477. doi: 10.1007/s005340200058. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Takeda T, Sakon M, Tsujimoto M, Higashiyama S, Noda K, Miyoshi E, Monden M, Matsuura N. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer. 2001;84:1377–1383. doi: 10.1054/bjoc.2000.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–1799. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- 15.Chen TH, Tseng LM, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, Hsia CY, Wu CW. Clinicopathologic and prognostic differences between patients with hepatitis B- and C-related resectable hepatocellular carcinoma. J Formos Med Assoc. 2001;100:443–448. [PubMed] [Google Scholar]
- 16.Mino M, Lauwers GY. Pathologic spectrum and prognostic significance of underlying liver disease in hepatocellular carcinoma. Surg Oncol Clin N Am. 2003;12:13–24. doi: 10.1016/s1055-3207(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 17.Chen GG, Merchant JL, Lai PB, Ho RL, Hu X, Okada M, Huang SF, Chui AK, Law DJ, Li YG, et al. Mutation of p53 in recurrent hepatocellular carcinoma and its association with the expression of ZBP-89. Am J Pathol. 2003;162:1823–1829. doi: 10.1016/S0002-9440(10)64317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii Y, Nakasato Y, Kobayashi S, Yamazaki Y, Aoki T. A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J Exp Clin Cancer Res. 2003;22:461–470. [PubMed] [Google Scholar]
- 19.Kuyvenhoven JP, van Hoek B, Blom E, van Duijn W, Hanemaaijer R, Verheijen JH, Lamers CB, Verspaget HW. Assessment of the clinical significance of serum matrix metalloproteinases MMP-2 and MMP-9 in patients with various chronic liver diseases and hepatocellular carcinoma. Thromb Haemost. 2003;89:718–725. [PubMed] [Google Scholar]
- 20.Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer. 2002;95:2346–2352. doi: 10.1002/cncr.10963. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Luo J, Zhang X, Hu J, Hui H, Wang C, Stern A. Inhibition of cell proliferation in HCC-9204 hepatoma cells by a c-myc specific ribozyme. Cancer Gene Ther. 2000;7:407–412. doi: 10.1038/sj.cgt.7700127. [DOI] [PubMed] [Google Scholar]
- 22.King KL, Hwang JJ, Chau GY, Tsay SH, Chi CW, Lee TG, Wu LH, Wu CW, Lui WY. Ki-67 expression as a prognostic marker in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1998;13:273–279. doi: 10.1111/j.1440-1746.1998.01555.x. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y, Matsuura N, Sakon M, Takeda T, Umeshita K, Nagano H, Nakamori S, Dono K, Tsujimoto M, Nakahara M, et al. Both cell proliferation and apoptosis significantly predict shortened disease-free survival in hepatocellular carcinoma. Br J Cancer. 1999;81:747–751. doi: 10.1038/sj.bjc.6690758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao ZC, Zheng SS, Wan YL, Jia CK, Xie HY. The molecular mechanism underlying angiogenesis in hepatocellular carcinoma: the imbalance activation of signaling pathways. Hepatobiliary Pancreat Dis Int. 2003;2:529–536. [PubMed] [Google Scholar]
- 25.Dhar DK, Naora H, Yamanoi A, Ono T, Kohno H, Otani H, Nagasue N. Requisite role of VEGF receptors in angiogenesis of hepatocellular carcinoma: a comparison with angiopoietin/Tie pathway. Anticancer Res. 2002;22:379–386. [PubMed] [Google Scholar]
- 26.Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339–5345. [PubMed] [Google Scholar]
- 27.Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- 28.Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003;16:552–557. doi: 10.1097/01.MP.0000071841.17900.69. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Hu J, Cai J, Yang X, Yang Z. Vascular endothelial growth factor expression in serum of patients with hepatocellular carcinoma. Chin Med J (Engl) 2003;116:772–776. [PubMed] [Google Scholar]
- 30.Chao Y, Li CP, Chau GY, Chen CP, King KL, Lui WY, Yen SH, Chang FY, Chan WK, Lee SD. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol. 2003;10:355–362. doi: 10.1245/aso.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, Wong J. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227–235. doi: 10.1097/00000658-200102000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak BK, Shim HJ, Park ES, Kim SA, Choi D, Lim HK, Park CK, Chung JW, Park JH. Hepatocellular carcinoma: correlation between vascular endothelial growth factor level and degree of enhancement by multiphase contrast-enhanced computed tomography. Invest Radiol. 2001;36:487–492. doi: 10.1097/00004424-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, Tang ZY, Fan J, Wu ZQ, Li XM, Liu YK, Liu F, Sun HC, Ye SL. Expression of platelet-derived endothelial cell growth factor and vascular endothelial growth factor in hepatocellular carcinoma and portal vein tumor thrombus. J Cancer Res Clin Oncol. 2000;126:57–61. doi: 10.1007/s004320050009. [DOI] [PubMed] [Google Scholar]


