Abstract
AIM: To explore both the in vitro and in vivo effects of denbinobin against colon cancer cells and clarify its underlying signal pathways.
METHODS: We used COLO 205 cancer cell lines and nude mice xenograft model to study the in vitro and in vivo anti-cancer effects of denbinobin.
RESULTS: Denbinobin at concentration of 10-20 μmol/L dose-dependently suppressed COLO 205 cell proliferation by MTT test. Flow cytometry analysis and DNA fragmentation assay revealed that 10-20 μmol/L denbinobin treatment induced COLO 205 cells apoptosis. Western blot analysis showed that caspases 3, 8, 9 and Bid protein were activated by denbinobin treatment to COLO 205 cells accompanied with cytochrome c and apoptosis-inducing factor (AIF) translocation. Pretreatment of MEK 1 inhibitor (U10126), but not p38 inhibitor (SB203580) and JNK inhibitor (SP600125), reversed denbinobin-induced caspase 8, 9 and Bid activation in COLO 205 cells suggesting that extracellular signal-regulated kinase were involved in the denbinobin-induced apoptosis in COLO 205 cells. Significant regression of tumor up to 68% was further demonstrated in vivo by treating nude mice bearing COLO 205 tumor xenografts with denbinobin 50 mg/kg intraperitoneally.
CONCLUSION: Our findings suggest that denbinobin could inhibit colon cancer growth both in vitro and in vivo. Activation of extrinsic and intrinsic apoptotic pathways and AIF were involved in the denbinobin-induced COLO 205 cell apoptosis.
Keywords: Denbinobin, Colon cancer, Nude mice, Apoptosis, ERK pathway
INTRODUCTION
Colon cancer is one of the most prevalent malignances in the world. It ranked as the second cause of cancer death in the Western societies[1]. In Taiwan, colon cancer is the third most common form of lethal cancer[2]. Although surgical treatment of early stage colon cancer showed promising effect, the prognosis for those advanced staging patients is still not satisfactory, mostly owing to high local recurrent rate and metastasis. Therefore, finding new therapeutic strategies to improve survival rate in colon cancer patients are very important issues.
There are growing evidences that compounds of plant origin have the ability to treat or prevent cancer. For example, the Chinese herbal preparation called PC-SPES, a mixture consisting of extracts from eight herbs, has been used increasingly in prostate cancer[3] and demonstrated activity against androgen independent prostate cancer in a prospective, multicenter, randomized phase II study[4]. Another example was taxol, which was purified from the stem bark of Taxus brevifolia and showed anti-tumorigenesis effect on breast and ovarian cancers[5]. Other pure compounds isolated from herb could provide novel therapeutic advantage in the treatment of cancers. For example, solamargine, a pure compound isolated from Solanum incanum herb, could be a potential drug for cisplatin-resistant human lung cancer cells[6] and beta-elemene, a wide spectrum anticancer drug derived from the Chinese herb Curcuma chaeocaulis, could reverse adriamycin-induced resistance in human breast cancer cell line[7]. Therefore, it is reasonable for us to search useful anti-cancer drugs from the traditional herbal medicine.
Previous study had demonstrated that denbinobin isolated from Dendrobium nobile, Ephemerantha lonchophylla, and E. fimbriata exerted potential anti-inflammatory, antioxidant, and anti-tumorigenesis effect[8-10]. Denbinobin was found to be cytotoxic against A549 (human lung carcinoma), SK-OV-3 (human ovary adenocarcinoma), and HL-60 (human promyelocytic leukemia) cell lines[9]. However, the anti-cancer effect of denbinobin and its underlying molecular mechanisms remain obscured. The purpose of this study is to explore the in vitro and in vivo anti-tumorigenesis effects of denbinobin against colon cancer and clarify its underlying molecular mechanisms.
MATERIALS AND METHODS
Cell lines and cell culture
The COLO 205 cell line was isolated from human colon adenocarcinoma (American Type Culture Collection CCL-222). The cells were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin (100 U/mL), streptomycin (100 mg/mL), and 0.3 mg/mL glutamine in a humidified incubator (37 °C, 50 mL/L CO2). Denbinobin (Pharmaceutical Industry, Technology and Development Center, Taiwan) was added at the indicated doses in 0.1% dimethylsulfoxide (DMSO). For control specimens, the same volume of DMSO was added in a final concentration of 1 mL/L without denbinobin.
MTT assay
COLO 205 cells were seeded in a 96-well plate at a density of 1×104 cells/well and allowed to adhere overnight. After removing the medium, 200 μL of fresh medium per well, containing 10 mmol/L Hepes (pH 7.4) was then added. Then, 50 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was added to the wells and the plate was incubated for 2-4 h at 37 °C in the dark. The medium was removed and 200 μL DMSO and 25 μL Sorensen’s glycine buffer was added to the wells. Absorbance was measured using an ELISA plate reader at 570 nm.
Drug treatment, and flow cytometry analysis
At 24 h after plating of cells, cells were washed thrice with phosphate-buffered saline (PBS) and then added with medium containing 10% FCS with various concentrations of denbinobin in a final concentration of 1 mL/L DMSO. The cell cycle stages in the denbinobin and DMSO-treated groups were measured by fluorescence-activated cell sorter (FACS) analysis. Cells were harvested and stained with propidium iodide (50 μg/mL) (Sigma Chemical Co., St. Louis, MO), and DNA content was measured using a FACScan laser flow cytometer analysis system (Becton-Dickinson, San Jose, CA); and 15000 events were analyzed for each sample.
Analysis of DNA fragmentation
Analysis of DNA fragmentation was performed as previously described[11]. Briefly, the denbinobin and DMSO-treated cells were seeded on 100-mm dishes. The DNA was extracted twice with equal volumes of phenol and once with chloroform-isoamyl alcohol (24:1 v:v), then precipitated with 0.1 volume of sodium acetate, pH 4.8, and 2.5 volumes of ethanol at -20 °C overnight, and finally centrifuged at 13000 g for 1 h. Genomic DNA was quantitated, and equal amounts of DNA sample in each lane were electrophoresed in a 2% agarose gel. The DNA was visualized by ethidium bromide staining.
Western analysis
Cells were washed with cold PBS, lysed in Golden lysis buffer, and performed Western blotting as described previously[12]. Briefly, cell lysates were prepared, electrotransferred, immunoblotted with anti-bax, bcl-2, cytochrome c (Cyto c), and apoptosis-inducing factor (AIF) monoclonal antibody or anti-caspases 9, 3 and Bid, total Akt polyclonal antibody (1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA); or with anti-caspase 8 and phosphorylated Akt monoclonal antibody (1:1000 dilution, Cell Signaling Technology, Beverly, MA); or with anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (1:2000 dilution, Biogenesis, Kingston, NH), and then with anti-mouse or anti-rabbit or anti-goat IgG antibody conjugated to horseradish peroxidase (1:5000 dilution, Santa Cruz Biotechnology) and visualized bands using enhanced chemiluminescence kits (2 min, ECL, Amersham). The expression of GAPDH was used as the control for equal protein loading.
Subcellular fractionation
As previously described[13], COLO 205 cells were harvested in isotonic mitochondrial buffer (210 mmol/L mannitol, 70 mmol/L sucrose, 1 mmol/L EDTA and 10 mmol/L HEPES pH 7.5), supplemented with protease inhibitor cocktail Complete (Boehringer Mannheim), and homogenized for 40 strokes with a Dounce homogenizer. Samples were centrifuged at 500 r/min for 5 min at 4 °C to eliminate nuclei and unbroken cells. The resulting supernatant was centrifuged at 10000 g for 30 min at 4 °C to separate the heavy membrane pellet, and the resulting supernatant was stored as the cytosolic fraction.
Treatment of COLO 205-derived xenografts in vivo
As previously described[14], COLO 205 cells were grown in RPMI 1640 supplemented with 10% FCS as described above. Cells were harvested through two consecutive trypsinizations, centrifuged at 300 r/min for 5 min, washed twice, and resuspended in sterile PBS. Cells (5×106) in 0.2 mL were injected subcutaneously between the scapulae of each nude mouse (purchased from National Science Council Animal Center, Taipei, Taiwan). After transplantation, tumor size was measured using calipers and the tumor volume was estimated according to the formula tumor volume (mm3) = L×W2/2, where L is the length and W is the width[15]. Once tumors reached a mean size of 200 mm3, animals received intraperitoneal injections of either 25 μL DMSO or 50 mg/kg denbinobin thrice per week for 4 wk.
Statistical analysis
Results were expressed as mean±SE for each study. Data were analyzed by Student’s t-test or linear regression method; a P value of 0.05 or less was considered statistically significant.
RESULTS
Denbinobin suppressed colon cancer cell growth
We first examined the antiproliferative effect of denbinobin on the colon cancer cells. Treatment of the COLO 205 cells with 10 μmol/L denbinobin in a time course study showed significant inhibition of COLO 205 cell growth starting from 12 to 24 h by MTT test (Figure 1A). Dose-dependent study by MTT test further demonstrated that treatment of denbinobin (10-20 μmol/L) to COLO 205 for 24 h suppressed cancer cell growth (Figure 1B).
Figure 1.
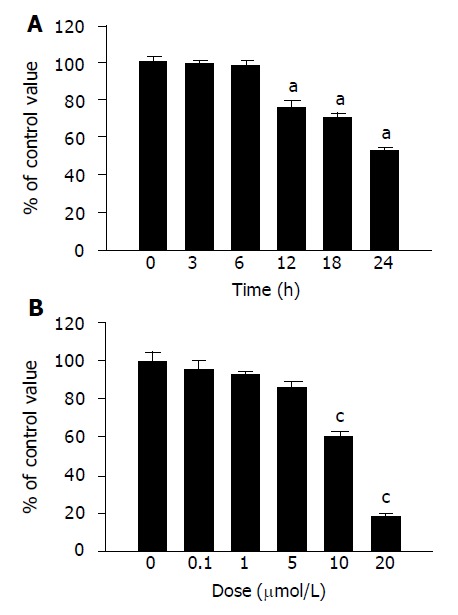
Effect of denbinobin on COLO 205 cell growth. COLO 205 cells were seeded in a 96-well plate until subconfluence and viable cells were determined by MTT assay. A: Time course study with 10 μmol/L denbinobin in 0.1% DMSO. Two independent experiments were performed in triplicate. aP<0.05 vs time 0. B: Dose-dependent study after treatment of denbinobin for 24 h. Two independent experiments were performed in triplicate. cP<0.05 vs DMSO control.
Denbinobin induced apoptosis
To further investigate the cellular mechanism of the denbinobin-induced growth inhibition, FACS analyses of DNA content in both DMSO- and denbinobin-treated COLO 205 cells were conducted. Figure 2A showed that 10-20 μmol/L denbinobin treatment to COLO 205 cells for 24 h induced a significant accumulation of cells at the sub-G1 phase of the cell cycle, suggesting that the observed growth inhibitory effect of denbinobin was due to induction of cell death. Further study by using DNA fragmentation assay also demonstrated that, at concentrations of 0-5 μmol/L denbinobin, apoptosis was not observed. However, when denbinobin concentration was increased to 10-20 μmol/L, apoptosis was observed in COLO 205 cells (Figure 2B).
Figure 2.
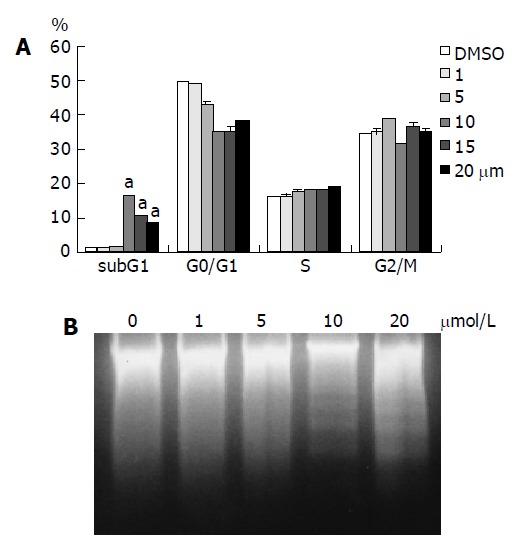
Treatment of COLO 205 cells with denbinobin (10-20 μmol/L) for 24 h induced apoptosis. A: FACS analysis of DNA content after 24-h incubation in culture medium supplemented with 10% FCS and 0.1% DMSO or 0-20 μmol/L denbinobin in 0.1% DMSO. Percentages of cells in sub-G1, G0/G1, S, and G2/M phases of the cell cycle determined using established CellFIT DNA analysis software were shown. Increased sub-G1 percentage of COLO 205 cells was shown after treatment with 10-20 μmol/L denbinobin. Two independent experiments were performed in triplicate. aP<0.05 vs DMSO control. B: Electrophoresis of genomic DNA from COLO 205 treated with denbinobin. A typical DNA ladder pattern associated with apoptosis was seen at 10-20 μmol/L denbinobin.
Denbinobin activated caspases 3, 8 and 9
Since it has been suggested[16] that the occurrence of apoptosis requires the activation of caspases, we investigated the involvement of caspase activation in the denbinobin-induced apoptosis in COLO 205 cells by Western blot analyses. Treatment of COLO 205 cells with 20 μmol/L denbinobin for 24 h induced caspase 3 activation evidenced by degradation of procaspase 3 bands (Figure 3A). To elucidate the apoptotic pathways involved in the activation of caspase 3, we examined the changes of caspases 8 and 9 protein levels in the denbinobin-treated COLO 205 cells. After treatment of COLO 205 cells with 20 μmol/L denbinobin for 12 h, activations of caspases 8 and Bid were evidenced by the degradation of the proenzymes of caspases 8 and cleavage of Bid proteins. Furthermore, increased expression of activation form of caspase 9 was noted after 18 h of 20 μmol/L denbinobin treatment to COLO 205 cells (Figure 3A).
Figure 3.
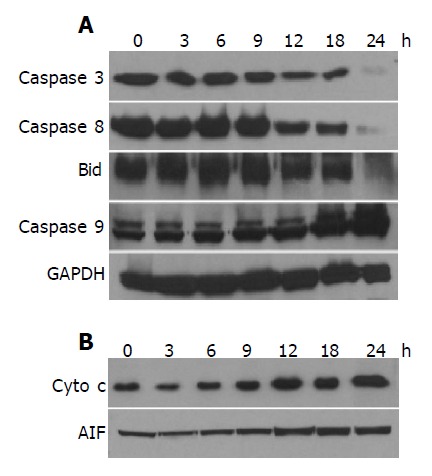
Effect of denbinobin on caspases, Bid protein levels or Cyto c and AIF translocation from mitochondria to cytosol. The whole cell proteins (A) or cytosolic proteins for Cyto c, AIF (B) were extracted from the cultured COLO 205 cells, which had been grown in 10% FCS and incubated for the indicated times with 0.1% DMSO or 20 μmol/L denbinobin in 0.1% DMSO. After electrophoresis, proteins were transferred onto Immobilon-P membranes, and then probed with proper dilutions of specific antibodies. Membranes were also probed with anti-GAPDH antibody to correct for difference in protein loading. Denbinobin time-dependently induced the activation of caspases and Bid (A), translocation of Cyto c and AIF (B).
Denbinobin induced Cyto c and AIF release from mitochondria
It has been demonstrated that activation of caspase 9 occurred during the release of Cyto c from mitochondria[17]. To examine whether this occurs in the denbinobin-induced apoptosis in COLO 205 cells, Cyto c release was monitored at various times after 10 μmol/L denbinobin treatment. Figure 3B showed that denbinobin treatment resulted in a significant accumulation of Cyto c as well as AIF in the cytosol fraction of cell extracts (Figure 3B). This denbinobin-induced elevation of cytosolic Cyto c was observed at 12 h and peaked at 24 h after denbinobin treatment. Under the same conditions, apoptosis was not observed until 12 h after denbinobin treatment (Figure 1A) suggesting that translocation of Cyto c and AIF occurred in the denbinobin-treated COLO 205 cells beforehand, and then caspases 9 and 3 activation, and apoptosis followed thereafter.
Denbinobin activated Bid protein
Proteins of the bcl-2 family are also believed to be involved in the control of apoptosis[18]. Activation of Bid by caspase 8 links the extrinsic to the intrinsic apoptotic pathway through mitochondrial damages[19] by releasing Cyto c from mitochondria, and thereby initiate apoptosis. Bcl-2 directly or indirectly operates to prevent the release of Cyto c from mitochondria. On the other hand, bax can trigger mitochondria to release Cyto c from mitochondria, and thereby initiate apoptosis. Accordingly, we examined the changes of bcl-2 protein levels in denbinobin-treated COLO 205 cells. Treatment of COLO 205 cells with 10-20 μmol/L denbinobin caused a dose-dependent activation of caspases 3, 8, and 9 (Figure 4A) as well as Bid activation evidenced by decreased Bid protein and appearance of its cleavage product (Figure 4B). In contrast, bcl-2 protein levels were not changed significantly (Figure 4B). Surprisingly, proapoptotic bax protein showed decreased expression (Figure 4B). These findings suggest that Bid but not bcl-2 or bax participated in the denbinobin-induced COLO 205 cells apoptosis.
Figure 4.
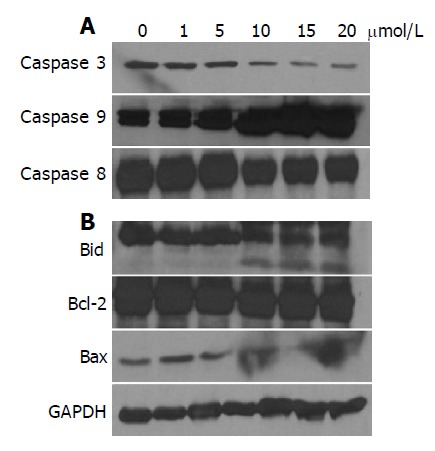
Effect of denbinobin on caspases (A) or Bid, bcl-2 and bax protein levels (B). The whole cell proteins were extracted from the cultured COLO 205 cells, which had been grown in 10% FCS and incubated for the indicated times with 0.1% DMSO or 20 μmol/L denbinobin in 0.1% DMSO. After electrophoresis, proteins were transferred onto Immobilon-P membranes, and then probed with proper dilutions of specific antibodies. Membranes were also probed with anti-GAPDH antibody to correct for difference in protein loading. Denbinobin dose-dependently induced the activation of caspases (A), Bid protein activation and decreased expression of bax (B).
Denbinobin-induced apoptosis involved extracellular signal-regulated kinase (ERK) pathway
To further study the signaling pathways involved in the denbinobin-induced COLO 205 cell apoptosis, the following experiments were performed. Since the extracellular signal-regulated kinase (ERK), p38 and JNK pathways have been suggested to be involved in the regulation of apoptotic processes, we applied an MEK 1 inhibitor (U10126), a p38 MAP kinase (p38 MAPK) inhibitor (SB203580) and a JNK inhibitor (SP600125) to examine whether they also participated in the denbinobin-induced COLO 205 cell apoptosis. As illustrated in Figure 5A, pretreatment of COLO 205 cells with 100 nmol/L U10126, but not SB20358 (20 μmol/L) and SP600125 (100 nmol/L), blocked the denbinobin-induced caspases 8, 9 and Bid protein activation in COLO 205 cell, suggesting that the ERK, but not p38 and JNK pathways, was involved in this process.
Figure 5.
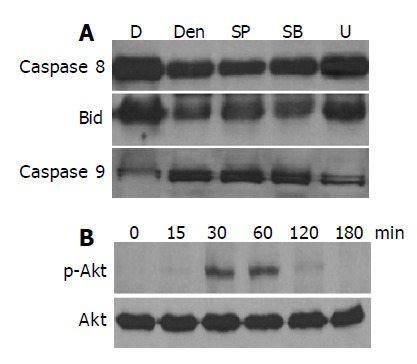
Effect of MAPK and Akt pathways in the denbinobin-induced COLO 205 cells apoptosis. A: Cells were pretreated with various kinds of MAPKs inhibitors; U10126 (U, 100 nmol/L), SB203580 (SB, 20 μmol/L), or SP600125 (SP, 100 nmol/L) for 1 h. The whole cell proteins were extracted from the cultured COLO 205 cells, which had been grown in 10% FCS and incubated for the indicated times with 0.1% DMSO or 20 μmol/L denbinobin in 0.1% DMSO for 24 h. After electrophoresis, proteins were transferred onto Immobilon-P membranes, and then probed with proper dilutions of specific antibodies; B: Effect of Akt pathway on the denbinobin treatment to COLO 205 cells. Time course study of 20 μmol/L denbinobin-treated COLO 205 cells was used to detect the total Akt proteins and the phorphorylated Akt by Western blot.
The serine/threonine kinase Akt is important for anti-apoptotic signaling pathways for normal cells. Accordingly, we tested whether Akt activity was regulated by denbinobin treatment to COLO 205 cells. Time course study in Figure 5B demonstrated that treatment of denbinobin to COLO 205 cells induced activation of Akt evidenced by increased phosphorylated form of Akt peaked from 30 to 60 min after treatment. Pretreatment of PI3K-Akt pathway inhibitor (Wortmannin, 100 nmol/L) failed to affect denbinobin-induced caspases activation (data not shown). These findings suggested that Akt pathway might not be directly involved in the denbinobin-induced apoptosis in COLO 205 cells.
Denbinobin inhibited colon tumor growth in vivo
We further examined the anti-tumor effect of denbinobin in vivo by treating athymic mice bearing COLO 205 tumor xenografts. After establishment of palpable tumors (mean tumor volume, 200 mm3), animals received denbinobin at dosage of 50 mg/kg or DMSO (control) thrice a week. A reduction in tumor volume between denbinobin-treated vs the DMSO-treated control group was detected by d 15. This difference became progressively more conspicuous, with the average tumor volume in the denbinobin-treated mice 32% that of the DMSO-treated mice after 4 wk of treatment (Figure 6A). In mice receiving these treatment regimens, no significant difference of body weight was noted (Figure 6B) and no gross signs of toxicity were observed (visible inspection of general appearance, and microscopic examination of individual organs) (data not shown). Our results indicated that denbinobin might be a potential useful chemotherapeutic agent in treating colon tumor.
Figure 6.
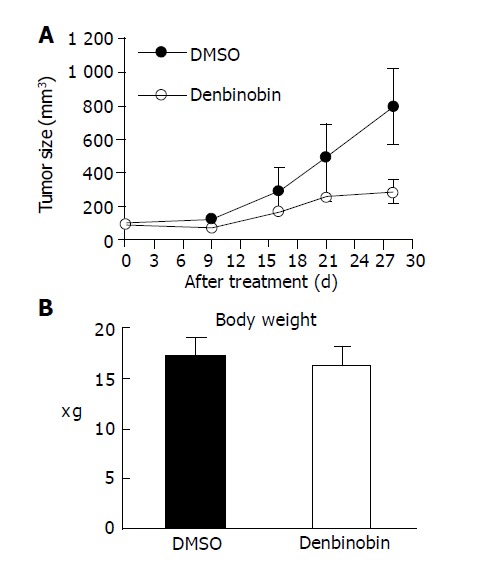
Growth of COLO 205 tumor xenografts in nude mice was suppressed by denbinobin treatment. Athymic nude mice injected with COLO 205 cells into subcutaneous tissue of inter-scapular area. Once tumor volume reached approximately 200 mm3, the animal received treatment of 50 mg/kg denbinobin or DMSO intraperitoneally thrice per week for 4 wk. (A) Average tumor volume (B) body weight of DMSO-treated (n = 5, filled circles) vs denbinobin-treated (n = 4, open circles) nude mice. Samples were analyzed in each group, and values represent the mean±SE. Comparisons were subjected to linear regression method in (A) and showed significant difference between DMSO vs denbinobin treatment group.
DISCUSSION
Denbinobin is one of the pure compounds isolated from the stems of D. moniliforme, known as Shi-Hu in Chinese medicine, which has been used for a long time to treat respiratory tract infection, fever and heat stroke[10]. Denbinobin has been shown to suppress the lipopolysaccharide-induced TNF-α formation in mouse macrophage cells suggesting that it exerts potent anti-inflammatory effect[10]. Chemical compounds with anti-inflammatory activity has been shown to protect against chemical toxicity and cancer[20,21]. In our in vitro study, we demonstrated that denbinobin at concentrations of 10-20 μmol/L inhibited the growth of COLO 205 cells in a dose-dependent manner (Figure 1). These results were not due to inhibition of cell division and indicated that there was an induction of apoptosis by denbinobin in the subcultured cancer cells (Figure 2). In vivo studies showed that intraperitoneal administration of denbinobin caused a striking and substantial regression of the COLO 205 tumor in nude mice (Figure 6). To our knowledge, this is the first demonstration that denbinobin inhibits the growth of colon cancer cells both in vitro and in vivo.
Apoptosis is a cell suicide mechanism that requires specialized cellular machinery. A central component of this machinery is a proteolytic system involving caspases, a highly conserved family of cysteine proteinases with specific substrates[16]. By Western blot analysis, we demonstrated that 10-20 μmol/L denbinobin induces activations of caspases 8 and 9 in COLO 205 cells, evidenced by decreases in the stainable procaspases 8, and increases in the active form of caspase 9 enzyme expression (Figures 3A and 4). Activation of caspases 8 and 9 can cleave and activate downstream caspases such as caspase 3 (Figures 3A and 4), which eventually leads to apoptosis[22].
Bcl-2 family proteins have been suggested to be involved in the regulation of apoptosis by controlling the release of Cyto c from mitochondria[18]. Activation of Bid by caspase 8 links the extrinsic to the intrinsic apoptotic pathway through mitochondrial damages[19]. Bcl-2 prevents apoptosis by blocking the release of Cyto c from mitochondria[23]. Bax, on the other hand, directly induces Cyto c release from mitochondria, and thereby triggers caspase 9 activation[24]. The results of the present study demonstrate that activation of Bid protein by denbinobin treatment in COLO 205 cells seems to be responsible for stimulating the release of Cyto c (Figure 3).
One of the major challenges in the cancer treatment is that many tumor cells carry mutations in key apoptotic genes such as p53, Bcl family proteins or those affecting caspase signaling. These defects resulted in failure of traditional chemotherapeutic treatments. Therefore, it is important to search for caspase-independent cell death mechanism in tumor[25]. It has been discovered that, in response to apoptotic stimuli, mitochondria can also release caspase-independent cell death effector such as AIF[26]. AIF is a phylogenetically ancient mitochondrial intermembrane flavoprotein endowed with the unique capacity to induce caspase-independent peripheral chromatin condensation and large-scale DNA fragmentation when added to purified nuclei[27]. In our study, 20 μmol/L denbinobin treatment induced the release of AIF from mitochondria to cytosol. These findings suggest a potential therapeutic advantage of denbinobin in the treatment of colon cancer.
Mitogen-activated protein (MAP) kinases, including ERK, c-Jun NH2-terminal kinases (JNK) and p38 MAPK are important intermediates of the signal-transduction pathway associated with proliferation or apoptosis[28]. A report has shown that ursodeoxycholic acid could induce colon cancer cell apoptosis through modulation of ERK pathway. Our results demonstrated that MEK 1 inhibitor but not JNK or p38 inhibitors could reverse the denbinobin-induced caspases 8, 9 and Bid activation (Figure 5A) suggesting that ERK pathway was involved in the denbinobin-mediated apoptosis in COLO 205 cells.
Akt promotes cell survival by inhibiting proapoptotic proteins such as Bad, forkhead, and p53, and activating pro-survival proteins such as NF-κB. It is likely that hyperactivity of the PI3K-Akt pathway is a selected mechanism for tumor cells to overcome apoptotic stimuli. In our results, we demonstrated that Akt was not suppressed but transient activated after denbinobin treatment to COLO 205 cells (Figure 5B) and pretreatment of Akt inhibitor has no effect on the denbinobin-induced COLO 205 cell apoptosis. These findings suggest that Akt pathway was not primarily regulated by denbinobin, instead its activation might be a secondary effect.
Our findings introduced our basic observations that denbinobin, a pure compound derived from Chinese herb, could induce apoptosis in colon cancer cells and suppress colon cancer growth in tumor bearing nude mice. The results from the present in vitro and in vivo studies highlight the molecular mechanisms of denbinobin-induced apoptosis in COLO 205 cells, which might have potential applications in the treatment of human colon cancer.
Footnotes
Supported by the Shin Kong Wu Ho-Su Memorial Hospital (SKH-TMU-93-16) and the Chi Mei Medical Center (93CM-TMU-09)
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Wei SC, Su YN, Tsai-Wu JJ, Wu CH, Huang YL, Sheu JC, Wang CY, Wong JM. Genetic analysis of the APC gene in Taiwanese familial adenomatous polyposis. J Biomed Sci. 2004;11:260–265. doi: 10.1007/BF02256569. [DOI] [PubMed] [Google Scholar]
- 3.Fu Y, Hsieh TC, Guo J, Kunicki J, Lee MY, Darzynkiewicz Z, Wu JM. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2004;322:263–270. doi: 10.1016/j.bbrc.2004.07.094. [DOI] [PubMed] [Google Scholar]
- 4.Oh WK, Kantoff PW, Weinberg V, Jones G, Rini BI, Derynck MK, Bok R, Smith MR, Bubley GJ, Rosen RT, et al. Prospective, multicenter, randomized phase II trial of the herbal supplement, PC-SPES, and diethylstilbestrol in patients with androgen-independent prostate cancer. J Clin Oncol. 2004;22:3705–3712. doi: 10.1200/JCO.2004.10.195. [DOI] [PubMed] [Google Scholar]
- 5.Ozols RF. Paclitaxel (Taxol)/carboplatin combination chemotherapy in the treatment of advanced ovarian cancer. Semin Oncol. 2000;27:3–7. [PubMed] [Google Scholar]
- 6.Liang CH, Liu LF, Shiu LY, Huang YS, Chang LC, Kuo KW. Action of solamargine on TNFs and cisplatin-resistant human lung cancer cells. Biochem Biophys Res Commun. 2004;322:751–758. doi: 10.1016/j.bbrc.2004.07.183. [DOI] [PubMed] [Google Scholar]
- 7.Hu J, Jin W, Yang PM. Reversal of resistance to adriamycin in human breast cancer cell line MCF-7/ADM by beta-elemene. Zhonghua ZhongLiu ZaZhi. 2004;26:268–270. [PubMed] [Google Scholar]
- 8.Chen HY, Shiao MS, Huang YL, Shen CC, Lin YL, Kuo YH, Chen CC. Antioxidant principles from Ephemerantha lonchophylla. J Nat Prod. 1999;62:1225–1227. doi: 10.1021/np990025f. [DOI] [PubMed] [Google Scholar]
- 9.Lee YH, Park JD, Baek NI, Kim SI, Ahn BZ. In vitro and in vivo antitumoral phenanthrenes from the aerial parts of Dendrobium nobile. Planta Med. 1995;61:178–180. doi: 10.1055/s-2006-958043. [DOI] [PubMed] [Google Scholar]
- 10.Lin TH, Chang SJ, Chen CC, Wang JP, Tsao LT. Two phenanthraquinones from Dendrobium moniliforme. J Nat Prod. 2001;64:1084–1086. doi: 10.1021/np010016i. [DOI] [PubMed] [Google Scholar]
- 11.Lin SY, Chang YT, Liu JD, Yu CH, Ho YS, Lee YH, Lee WS. Molecular mechanisms of apoptosis induced by magnolol in colon and liver cancer cells. Mol Carcinog. 2001;32:73–83. doi: 10.1002/mc.1066. [DOI] [PubMed] [Google Scholar]
- 12.Lin SY, Liang YC, Ho YS, Tsai SH, Pan S, Lee WS. Involvement of both extracellular signal-regulated kinase and c-jun N-terminal kinase pathways in the 12-O-tetradecanoylphorbol-13-acetate-induced upregulation of p21(Cip1) in colon cancer cells. Mol Carcinog. 2002;35:21–28. doi: 10.1002/mc.10070. [DOI] [PubMed] [Google Scholar]
- 13.Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003;22:4385–4399. doi: 10.1093/emboj/cdg423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH, Lee WS. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem. 2002;84:532–544. [PubMed] [Google Scholar]
- 15.Osborne CK, Coronado EB, Robinson JP. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur J Cancer Clin Oncol. 1987;23:1189–1196. doi: 10.1016/0277-5379(87)90154-4. [DOI] [PubMed] [Google Scholar]
- 16.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 17.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 18.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q, Kinneer K. Chemoprotection by phenolic antioxidants. Inhibition of tumor necrosis factor alpha induction in macrophages. J Biol Chem. 2002;277:2477–2484. doi: 10.1074/jbc.M106685200. [DOI] [PubMed] [Google Scholar]
- 21.Na HK, Surh YJ. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands as bifunctional regulators of cell proliferation. Biochem Pharmacol. 2003;66:1381–1391. doi: 10.1016/s0006-2952(03)00488-x. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 24.Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–2796. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- 27.Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 28.Tominaga K, Higuchi K, Sasaki E, Suto R, Watanabe T, Fujiwara Y, Oshitani N, Matsumoto T, Kim S, Iwao H, et al. Correlation of MAP kinases with COX-2 induction differs between MKN45 and HT29 cells. Aliment Pharmacol Ther. 2004;20 Suppl 1:143–150. doi: 10.1111/j.1365-2036.2004.01986.x. [DOI] [PubMed] [Google Scholar]


