Abstract
AIM: To investigate the viral and host causes of fatty liver in chronic hepatitis B patients and the role of fat deposits in liver damage.
METHODS: A total of 164 patients (113 males and 51 females, average age 35±11.3 years, and range 10-62 years) with previously untreated chronic hepatitis B were included in the study. The patients were divided into two groups depending on the result of liver biopsy: group without steatosis (100 patients with <5% hepatosteatosis) and group with steatosis (64 patients with >5% hepatosteatosis). The groups were compared in terms of gender, body mass index (BMI), liver enzymes (ALT, AST, ALP, GGT), cholesterol, triglyceride, HBeAg, viral load, and histological findings. In the group with steatosis, the patients were subdivided depending on the degree of steatosis into mild group (45 patients with 5-24% steatosis), and severe group (19 patients with >25% steatosis).
RESULTS: In the group of chronic hepatitis B with steatosis, the mean age, BMI, cholesterol, and triglyceride levels were significantly higher than those in the group without steatosis (P<0.05). Steatosis was found in 53 (46.9%) of male patients and 11 (22%) of female patients (P<0.05). No significant difference was found in the positivity of ALT, AST, ALP, GGT, HBeAg, viral load, histological activity index (HAI) and stage between the two groups (P>0.05). In the group with severe steatosis, the BMI was significantly higher than that in the group with mild steatosis (P<0.05). No significant difference was found in the other parameters between the groups (P>0.05).
CONCLUSION: Steatosis in chronic hepatitis B appears to be a result of metabolic factors of the host rather than the effect of viruses. Steatosis is unrelated to the HAI and degree of fibrosis, which are considered as the histological indicators of liver damage.
Keywords: Chronic hepatitis B, Steatosis
INTRODUCTION
Hepatosteatosis is defined as fat deposition in the liver that exceeds 5% of the total weight of liver, or with more than 5% of hepatocytes containing fat deposits under light microscopic examination[1]. It occurs under several disease states. The most common causes are alcohol, metabolic diseases, drugs, and nutritional disorders.
Chronic hepatitis C and hepatosteatosis have been shown to occur together often. It is thought that the hepatosteatosis of chronic hepatitis C is associated with the effects of viruses. In these patients, fat deposition appears to be an augmenting factor in liver damage[2-5].
In the literature, few data is available on the role of chronic hepatitis B, which forms an important disease group in hepatology, in steatosis. Some hepatitis C viruses are termed steatovirus[6,7]. No important study investigating the relationship between chronic hepatitis B and fatty liver and the effect of steatosis on the course of the disease is available.
In this study, whether the cause of fatty liver in chronic hepatitis B patients who received no previous treatment is due to viral or host factors and the role of fat deposition in liver damage were investigated.
MATERIALS AND METHODS
The study included 164 patients in 1997-2002 with the diagnosis of chronic hepatitis B (positive for HBsAg, elevated transaminase levels for at least 6 mo, and histopathological findings) who received no antiviral treatment. The following patients were excluded from the study. Those receiving antiviral treatment before the study, those on hepatotoxic drug treatment, those consuming alcohol regularly or excessively, those diagnosed of cirrhosis, anti-HCV or anti-Delta positive patients, those diagnosed as having autoimmune or other metabolic liver diseases. The height and weight of all patients were determined and the body mass index (BMI) was calculated. Based on the BMI, the patients were classified as normal (BMI = 18.5-24.9), over weight (BMI = 25-29.9), obese (BMI = 30-34.9), or extremely obese (BMI = 35-39.9). Liver function, blood glucose, cholesterol and triglyceride levels of each patient were also determined. For each patient HBsAg, HBeAg (Abbot Laboratories, North Chicago, IL, USA) were measured and HBV DNA was determined by PCR (Amplicor, Roche). The viral load was measured by hybridization (Hybrid Capture Assay, Digene, USA) in 122 patients.
Liver biopsy was performed on each patient. Determination of the histological activity index (HAI) and staging of the biopsy materials were done according to the Knodell’s classification[8]. HAI was scored as portal inflammation (0-4), lobular degeneration (0-4), and periportal necrosis (0-10). According to the degree of fibrosis, staging was made from 0 to 4 (0: no fibrosis, I: mild, II: moderate, III: severe, IV: cirrhosis). In the classification of steatosis less than 5% was considered normal, 5-25% as mild, greater than 25% as severe fat deposition.
Statistical analysis
Kruskall-Wallis variance analysis, Mann-Whitney U tests and the χ2 tests were performed.
RESULTS
The 164 chronic hepatitis B patients included in the study were subdivided into group with and without steatosis according to the findings of the liver biopsy materials. One hundred patients had no steatosis, while 64 had steatosis. Fifty-one of the patients were females and 113 were males. The mean age was 35±11.3 years (range 10-62 years). Eleven of female patients (22%) and 53 (46.9%) of the male patients were found to have steatosis. The difference was statistically significant (χ2: 9.67; P<0.05).
The average age, BMI, cholesterol and triglyceride level in the group with steatosis were found to be significantly higher than those in the group without steatosis (P<0.05). The differences in the average AST, ALT, ALP, GGT and viral load values showed no statistical significance (P<0.05).
The group of patients without steatosis according to the degree of fibrosis showed that 30 (30%) patients were of stage 0, 50 (50%) of stage 1, 7 (7%) of stage 2, and 13 (13%) of stage 3. In the patients with steatosis, however, 24 (37.5%), 29 (45.3%), 3 (4.7%), and 8 (12.5%) of the patients were of stages 0, 1, 2, and 3, respectively. The difference between them was not statistically significant (χ2: 1.19; P>0.05). Examination of the group without steatosis according to the HAI revealed 16 (16%) patients to be of HAI 1 [Knodell score (KS) 0-3], 48 (48%) of HAI 2 (KS 4-8), 23 (23%) of HAI 3 (KS 9-12) and 13 (13%) of HAI 4 (KS 13-18). In the group with steatosis, however, the corresponding number was 7 (10.9%), 34 (53.1%), 16 (25%), and 7 (10.9%), respectively. The differences between the groups were not statistically significant (χ2: 1.12; P>0.05).
Of the 164 patients, 106 were found to be HBeAg (-) and 58 HBeAg (+). Steatosis was discovered in 17 (29.3%) of those with HBeAg (+) and in 46 (44.3%) of those with HBeAg (-). The difference between the two groups was not statistically significant (χ2: 3.55; P>0.05). The findings are summarized in Tables 1, 2 and 3 and in Figure 1.
Table 1.
Comparison of groups according to demographic features.
| Steatosis (–) n = 100 | Steatosis (+) n = 64 | P | |
| Age (yr) | 33.23±11.70 | 37.90±10.09 | 0.006a |
| BMI | 25.25±3.93 | 27.75±3.70 | 0.000a |
| Sex (female, %) | 40 (78) | 11 (22) | 0.008a |
| (male, %) | 60 (53.1) | 53 (46.9) |
P<0.05 difference is statistically significant.
Table 2.
Comparison of groups according to biochemical features.
| Steatosis (–) n = 100 | Steatosis (+) n = 64 | P | |
| AST | 76.50±56.14 | 70.04±54.61 | 0.469 |
| ALT | 131.62±109.39 | 113.45±68.19 | 0.686 |
| GGT | 39.67±38.27 | 44.29±35.33 | 0.139 |
| ALP | 175.08±75.53 | 173.39±65.68 | 0.834 |
| Cholesterol | 162.20±38.58 | 184.89±34.54 | 0.000a |
| Triglyceride | 102.50±44.66 | 133.67±65.96 | 0.004a |
P<0.05 difference is statistically significant.
Table 3.
Comparison of groups according to virological and histopathological features.
| Steatosis (-) n = 100, % | Steatosis (+) n = 64, % | P | |
| HBeAg (–) | 58 (58.6) | 46 (73) | 0.059 |
| (+) | 42 (41.4) | 17 (27) | |
| HBV DNA (–) | |||
| (+) | 9 (12.2) | 12 (25) | 0.067 |
| 65 (87.8) | 36 (75) | ||
| Viral load (pg/mL) | 1628.24±2944.29 | 781.55±1292.36 | 0.063 |
| Stage 0 | 30 (30) | 24 (37.5) | |
| 1 | 50 (50) | 29 (45.3) | 0.754 |
| 2 | 7 (7) | 3 (4.7) | |
| 3 | 13 (13) | 8 (12.5) | |
| HAI 0–3 | 16 (16) | 7 (10.9) | |
| 4–8 | 48 (48) | 34 (53.1) | 0.772 |
| 9–12 | 23 (23) | 16 (25) | |
| 13–17 | 13 (13) | 7 (10.9) |
Figure 1.
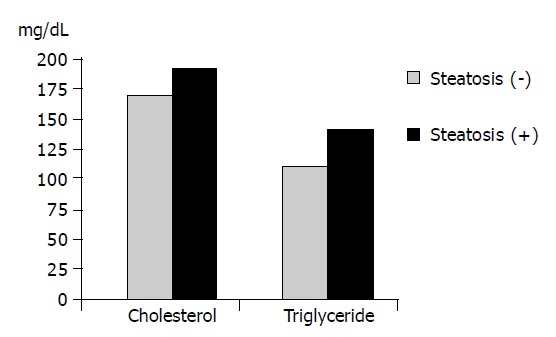
Comparison of cholesterol and triglyceride levels of groups with and without steatosis (P<0.05).
The presence of diabetes mellitus or a history of alcohol was considered as a risk factor in the patients. Based on this, 23 patients were found to carry the risk factor, 141 patients did not carry this risk.
Although steatosis was found in 12 (52.2%) patients among those with risk factors, and in 52 (36.9%) of those without the risk factors, the difference between the groups, however, was not statistically significant (χ2: 1.94; P>0.05).
The 164 patients admitted into the study were subdivided into three groups depending on the biopsy findings: group without steatosis (<5%), group with mild steatosis (5-25%), and group with advanced degree steatosis (>25%). There were 100 patients without steatosis, 45 with mild steatosis, and 19 with advanced degree steatosis. Comparison of the three groups in terms of the mean AST, ALT, ALP, GGT and viral load values showed no statistically significant differences (P>0.05). In terms of the mean age, BMI, cholesterol and triglyceride levels, statistically significant differences were observed between the groups (P<0.05). Comparison of the groups in pairs revealed significantly higher values in BMI and cholesterol levels in the group with mild steatosis than in those without steatosis (P<0.05). In the group with advanced degree steatosis, the BMI, triglyceride and cholesterol levels were found to be significantly higher than those in the group without steatosis (P<0.05). The BMI in the group with advanced degree steatosis was found to be significantly higher than that in the group with mild steatosis (P<0.05, Figure 2).
Figure 2.
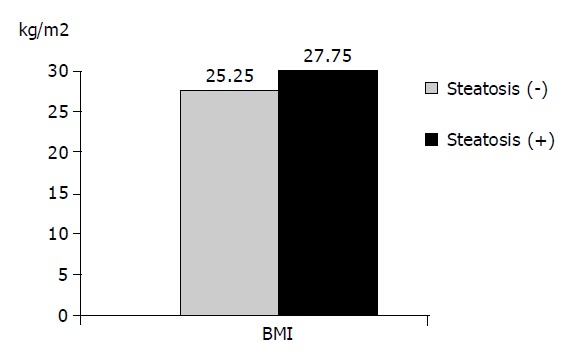
Comparison of BMI of groups with and without steatosis (P<0.05).
DISCUSSION
Viral hepatitis steatosis is thought to be associated with chronic hepatitis C. Steatosis in chronic hepatitis C has been postulated to be associated with the effects of the viruses rather than an immunological response. We, therefore, in our own study, tried to investigate the relationship between chronic hepatitis B virus, another viral agent causing chronic liver diseases, and steatosis. In the end, it was concluded that steatosis in chronic hepatitis B was independent of the viral effect.
In chronic liver diseases other than chronic hepatitis C, no study showing the relationship between steatosis and the disease is available. In these diseases steatosis has been thought to be a different independent pathological entity[9,10]. A study reports that chronic hepatitis B has occurred concurrently with steatosis in 27% of patients[11]. In another study, patients with hepatitis C and B in terms of risk factors were compared, and steatosis was found to occur more frequently in chronic hepatitis C[12]. Unfortunately, the number of chronic hepatitis B patients with steatosis in this study was rather very small.
Some studies, demonstrating the association between steatosis and chronic hepatitis B, described the viruses as “fibroviruses” in some patients and as “steatoviruses” in others[6,7]. One of these studies included patients with relapse of chronic hepatitis B after liver transplantation. This is an interesting study in view of the fact that it was able to compare the histopathological findings of uninfected liver before transplantation and after exposure to the viruses after transplantation[6].
In our study genotyping of the virus could not be conducted due to the combined economic constraints, inadequate laboratory facilities and the fact that this procedure was not yet done routinely. It has been reported that chronic hepatitis C steatosis is associated with some HCV genotypes[2]. There could have been the chance of defining some of the viruses as exhibiting “steatoviruses” based on genotyping.
Steatosis often occurs in some conditions and diseases such as obesity, hyperlipidemia, alcohol consumption and diabetes mellitus. In this study, steatosis was found to be associated with obesity and hyperlipidemia. The BMI was the only factor that was related to the degree of steatosis in a linear fashion. Whereas in some studies this linear relationship between the BMI and degree of steatosis was mentioned[13], other studies showed the opposite[14]. On this issue, the distribution of body fat rather than the quantity should be the determinant[15]. This might explain why steatosis was observed to be associated with the male gender in this study. It has been reported that steatosis is highly associated with the female gender[13,16]. Some recent studies describe equal rates[17]. In our study, the observation that steatosis showed an increase with advancing age is in agreement with what was reported in literature[18].
It was reported that steatosis was a promoting factor for liver damage in HCV infections[2,3]. The effect of steatosis on histopathological damage in chronic hepatitis B is unknown. In our study, no relationship was established between liver damage and steatosis in chronic hepatitis B.
In conclusion, steatosis in chronic hepatitis B seems to be a result of metabolic causes attributable to the host rather than the effect of the viruses.
References
- 1.Schiff ER, Sorell MF, Maddrey WC. Disease of the Liver. 6 th ed. Philedelphia: Lippincot- Williams and Wilkins; 1999. pp. 1185–1197. [Google Scholar]
- 2.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 3.Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215–1219. doi: 10.1002/hep.510290401. [DOI] [PubMed] [Google Scholar]
- 4.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837–842. doi: 10.1016/s0168-8278(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 5.Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM, Dhumeaux D. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut. 2003;52:288–292. doi: 10.1136/gut.52.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips MJ, Cameron R, Flowers MA, Blendis LM, Greig PD, Wanless I, Sherman M, Superina R, Langer B, Levy GA. Post-transplant recurrent hepatitis B viral liver disease. Viral-burden, steatoviral, and fibroviral hepatitis B. Am J Pathol. 1992;140:1295–1308. [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumaru K, Ishii K, Shinohara M, Sumino Y, Aikawa A, Hasegawa A, Nonaka H, Akima M. A renal allograft recipient with viral-burden, steatoviral, and fibroviral hepatitis B who achieved remission with Lamivudine. J Clin Gastroenterol. 2003;36:187–188. doi: 10.1097/00004836-200302000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;11:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 9.Samarasinghe D, Tasman-Jones C. The clinical associations with hepatic steatosis: a retrospective study. N Z Med J. 1992;105:57–58. [PubMed] [Google Scholar]
- 10.Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137–145. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824–1832. doi: 10.1016/0016-5085(93)91081-r. [DOI] [PubMed] [Google Scholar]
- 12.Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Host- and disease-specific factors affecting steatosis in chronic hepatitis C. J Hepatol. 1998;29:198–206. doi: 10.1016/s0168-8278(98)80004-4. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 14.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 15.Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710–723. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 17.George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 18.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]


