Abstract
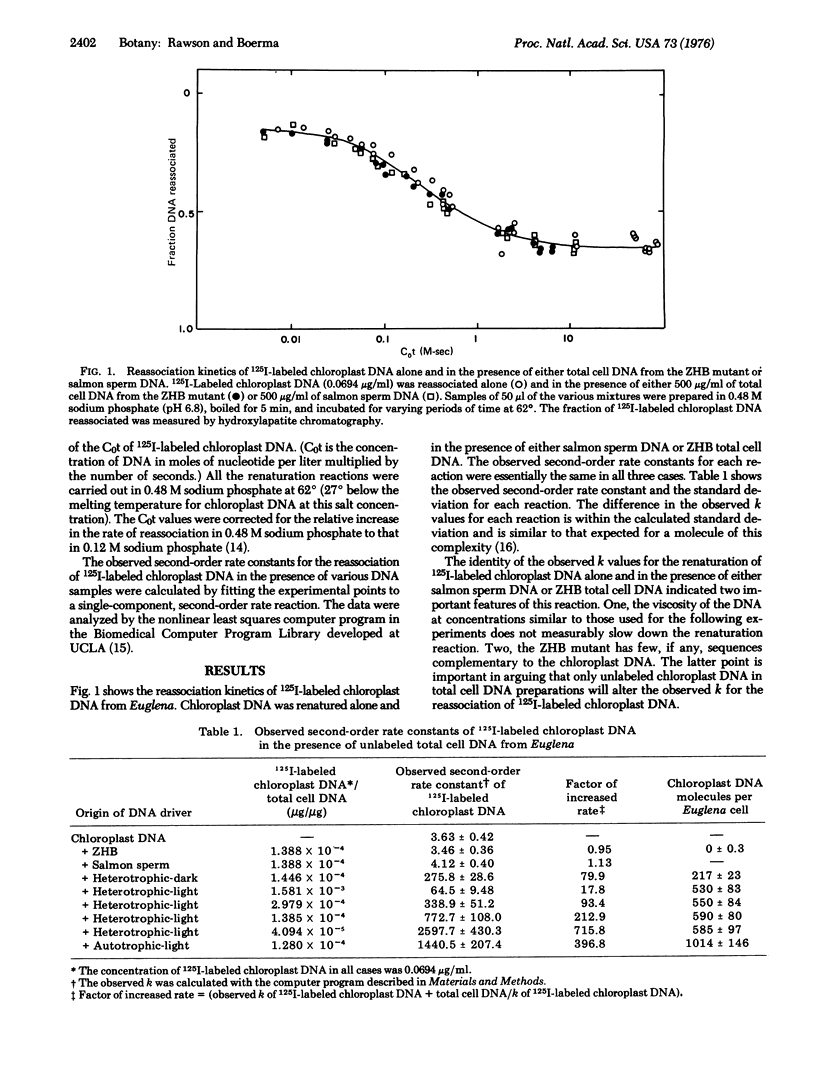
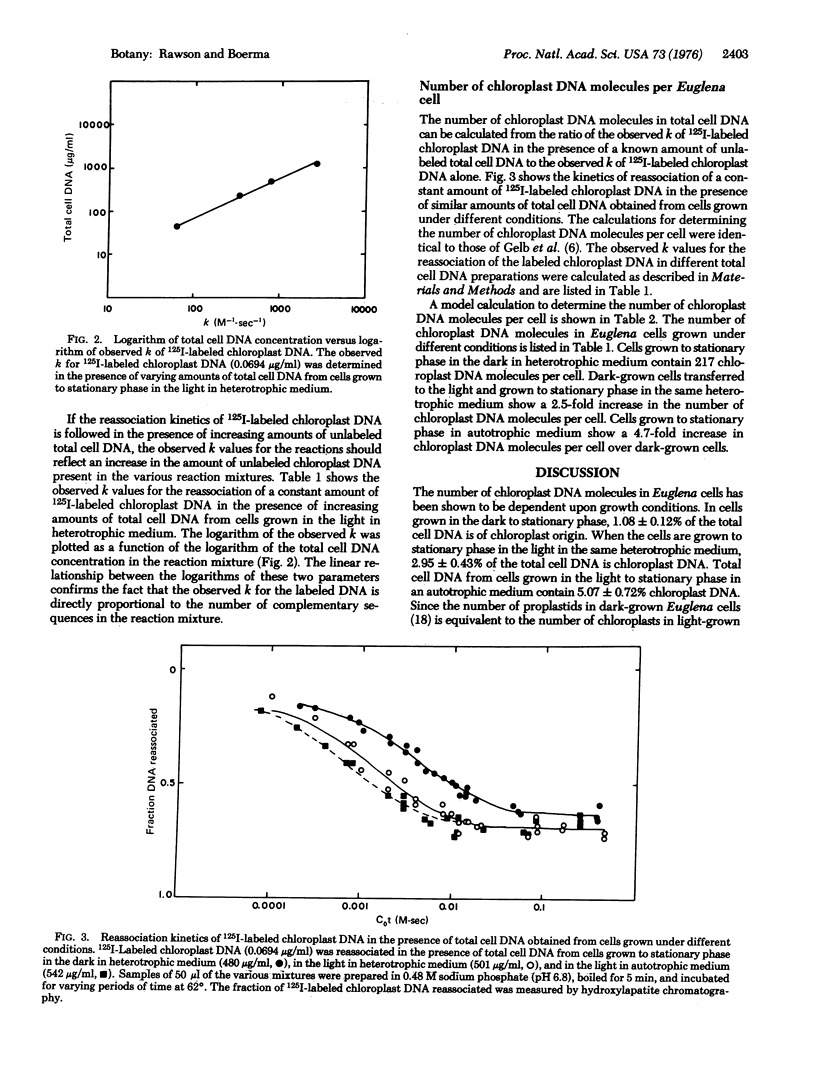
The number of chloroplast DNA molecules in Euglena gracilis cells was measured by determining the shift in the observed second-order rate constant for the reassociation of 125I-labeled chloroplast DNA in the presence of unlabeled total cell DNA. Cells grown to stationary phase in the dark contained 217 molecules of chloroplast DNA. Cells grown to stationary phase in the light in either heterotrophic or autotrophic medium contained 590 and 1014 chloroplast DNA molecules, respectively. The observed second-order rate constant for the reassociation of 125I-labeled chloroplast DNA was not significantly altered in the presence of total cell DNA from a heat-bleached mutant, ZHB, which lacks chloroplast DNA. This evidence suggests that there is less than 0.3 of a chloroplast DNA molecule present in the nucleus of Euglena.
Keywords: observed second-order rate constant
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Haselkorn R. Chloroplast RNA populations in dark-grown, light-grown, and greening Euglena gracilis. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2536–2539. doi: 10.1073/pnas.68.10.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. D., Haselkorn R. The isolation of Euglena gracilis chloroplasts uncontaminated by nuclear DNA. Biochim Biophys Acta. 1972 Jan 18;259(1):1–4. doi: 10.1016/0005-2787(72)90467-4. [DOI] [PubMed] [Google Scholar]
- Chelm B. K., Hallick R. B. Changes in the expression of the chloroplast genome of Euglena gracilis during chloroplast development. Biochemistry. 1976 Feb 10;15(3):593–599. doi: 10.1021/bi00648a022. [DOI] [PubMed] [Google Scholar]
- Edelman M., Cowan C. A., Epstein H. T., Schiff J. A. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA, VIII. CHLOROPLAST-ASSOCIATED DNA. Proc Natl Acad Sci U S A. 1964 Nov;52(5):1214–1219. doi: 10.1073/pnas.52.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Klein S., Schiff J. A., Holowinsky A. W. Events surrounding the early development of Euglena chloroplasts. II. Normal development of fine structure and the consequences of preillumination. Dev Biol. 1972 May;28(1):253–273. doi: 10.1016/0012-1606(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Richards O. C. Synthesis and turnover of Euglena gracilis nuclear and chlorplast deoxyribonucleic acid. Biochemistry. 1972 May 23;11(11):2036–2043. doi: 10.1021/bi00761a007. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Wolstenholme D. R., Ryan R. S., Hunter J. A., Richards O. C. Circular chloroplast DNA from Euglena gracilis. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1169–1173. doi: 10.1073/pnas.68.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Parenti F., Brawerman G., Preston J. F., Eisenstadt J. M. Isolation of nuclei from Euglena gracilis. Biochim Biophys Acta. 1969 Nov 19;195(1):234–243. doi: 10.1016/0005-2787(69)90620-0. [DOI] [PubMed] [Google Scholar]
- Rawson J. R., Boerma C. L. A measurement of the fraction of chloroplast DNA transcribed during chloroplast development in Euglena gracilis. Biochemistry. 1976 Feb 10;15(3):588–592. doi: 10.1021/bi00648a021. [DOI] [PubMed] [Google Scholar]
- Rawson J. R., Haselkorn R. Chloroplast ribosomal RNA genes in the chloroplast DNA of Euglena gracilis. J Mol Biol. 1973 Jun 15;77(1):125–132. doi: 10.1016/0022-2836(73)90366-5. [DOI] [PubMed] [Google Scholar]
- Rawson J. R. The characterization of Euglena gracilis DNA by its reassociation kinetics. Biochim Biophys Acta. 1975 Aug 21;402(2):171–178. doi: 10.1016/0005-2787(75)90036-2. [DOI] [PubMed] [Google Scholar]
- Richards O. C. Hybridization of Euglena gracilis chloroplast and nuclear DNA. Proc Natl Acad Sci U S A. 1967 Jan;57(1):156–163. doi: 10.1073/pnas.57.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]


