Abstract
AIM: To evaluate safety and possible efficacy of induction of oral immune regulation using colitis extracted proteins (CEP) in Crohn’s disease (CD) subjects.
METHODS: Ten CDs were treated orally with autologous CEP thrice weekly for 16 wk. Subjects were monitored for CDAI and IBDQ. Immune modulatory effect was assessed by T-lymphocyte FACS analysis, CEP-specific IFNγ ELISPOT assay and cytokine levels.
RESULTS: Induction of oral immune regulation significantly ameliorated disease activity. All (10/10) subjects had clinical response (CDAI ≤ 70) and 7/10 achieved clinical remission (CDAI ≤ 150). Significant increase in mean IBDQ score was noted (134±9 vs 164±12). No treatment-related adverse events were noted. High levels of CEP-specific IFNγ spot forming colonies were detected in five subjects prior to treatment and in all five, a marked decrease was observed. The CD4+/CD8+ lymphocyte ratio and peripheral NKT cell numbers increased significantly, in 7/10 and in 5/10 subjects, respectively. Significant increase in serum IL-10 and IL-4 levels was observed in 7/10 subjects during treatment period.
CONCLUSION: Immune regulation via oral administration of CEP is a safe and possibly effective treatment for subjects with moderate CD and may provide means of antigen-specific immune modulation.
Keywords: Crohn’s disease, Colitis extracted proteins
INTRODUCTION
Crohn’s disease (CD) is an immune mediated disorder. Both an appropriate response to an injurious, exogenous agent, or abnormal host responses to ubiquitous agents were suggested to play a role in the pathogenesis of the disease[1,2]. Abnormalities of T-cell-mediated immunity, including cutaneous anergy, and changes in T-cell subsets were described in these patients[1]. In addition, patients with CD have antibodies against components of colon cells and several different bacterial antigens. Changes in mucosal-cell-mediated immunity were identified, including alterations in mucosal T-cell subsets and concentrations of mucosal IgG cells, suggesting chronic antigen stimulation. Exposure of target antigens after infectious, immune, or toxic damage leads to activation of mucosal immune cells, resulting in cytokines that lead to mucosal inflammatory response[3-5]. An imbalance between Th1-pro-inflammatory and Th2-anti-inflammatory subtype of immune response plays a role in the pathogenesis of CD[6]. In both experimental colitis and in patients with CD, the disease is a Th1-mediated immune disorder resulting in a life-long inflammatory response against gut epitopes. Anti-inflammatory cytokines such as IL-10 downregulate the pro-inflammatory effects of Th1-mediated cytokines, thereby alleviating the disease[7-11].
Current treatments of CD, similar to that of other immune-mediated disorders, require overcoming the immune response towards disease-associated target antigens[12]. This involves systemic immunosuppression. New modes of immune modulatory treatments are not antigen specific, and therefore are either not effective in a large number of patients, or associated with unwanted side effects[12].
Oral immune regulation is a recognized procedure alteration of the host immune response towards orally administered antigens[13-15]. It was previously shown effective in preventing immune-mediated disorders in animal models[16-20]. This method was also tested in humans with various immune-mediated disorders[21]. Recently it has been shown by us and others that oral immune regulation can be used to alleviate experimental colitis in a model system using mice treated with 2,4,6-trinitrobenzene sulfonic acid (TNBS)[9,22-26]. TNBS induces an autoimmune response resembling CD in humans[22]. Oral administration of low doses of colitis-extracted proteins to mice with experimental colitis significantly decreased the inflammatory response.
The aims of the present study were to determine the safety and tolerability of oral administration of autologous colonic mucosal cells from CD patients, and to determine the efficacy of this mode of treatment in patients with CD. The effects of oral immune regulation towards colonic proteins on the immune system were studied. The results show that immune regulation via oral administration of proteins extracted from colonic mucosa was a safe and possibly an effective treatment for subjects with moderate to severe CD.
MATERIALS AND METHODS
Patients
Patient population One group of 10 patients was followed up in an open-labeled, non-randomized, one-center prospective trial. All experiments were carried out in accordance with the guidelines of the Hebrew-University-Hadassah Institutional Committee for Human Clinical Trials and Good Clinical Practice regulations. The Israel Ministry of Health Committee for Human Trials approved all experiments.
Inclusion criteria Eligible participants (men and women >18 years) signed a written informed consent. The diagnosis of CD with clinical evidence of active (symptomatic) disease was based on clinical history, blood tests and/or histology, or X-ray, or endoscopy, with a CDAI score >200 and <350. Subjects receiving oral steroid therapy were requested to have received a dose equivalent to or <25 mg of prednisone per day, and to have been on a stable dose for 2 wk prior to their first visit.
Exclusion criteria Subjects who had undergone bowel surgery within the last 3 mo; subjects who had a prior colostomy, ileostomy, or colectomy with ileorectal anastomosis; subjects likely to require emergency surgery for persistent intestinal obstruction, bowel perforation, uncontrolled bleeding or abdominal abscess or infection, toxic megacolon; subjects whose symptoms were believed to be largely due to the presence of fibrotic strictures; subjects with other infectious or neoplastic diseases of the bowel; subjects with an acute infectious disease; subjects receiving oral prednisone at a dose of >25 mg/d (or equivalent); subjects who were receiving an elemental diet or parenteral nutrition; subjects who were receiving immunosuppressive drugs such as azathioprine or 6-mercaptopurine; subjects who had either received methotrexate or cyclosporine or anti-TNFα or who had participated in another clinical trial within the last 3 mo; subjects with a history of major psychiatric disturbance, or who were drug or alcohol abusers; subjects with a history of GI tract malignancy or IBD-associated malignant changes in the intestine; subjects with a history of coagulopathy; women with child-bearing potential unless surgically sterile or using adequate contraception (either IUD, oral or depot contraceptive, or barrier plus spermicide); pregnant or breastfeeding mothers were excluded from the study.
Oral antigen preparation and administration Subjects who fulfilled the inclusion/exclusion criteria for participation in the study were scheduled for a colonoscopy during which time colon biopsies were taken from the subject for preparation of the colon-specific protein (study drug). The containing extract was prepared according to Enzo Therapeutics, Inc., Specification Number L0060-00-0109, “Preparation of Autologous Colon-Specific Antigen for Oral Immune Regulation for IBD: Crohn’s Disease”. Additional tissue samples were used for pathological analysis. Preliminary tests showed that each intestinal biopsy yielded approximately 300 μg protein. Thus, six biopsies produced approximately 1800 μg protein, which provided a supply of protein for the entire study. The administered dose based on previous studies in human subjects was between 18 and 30 μg/dose. Protein extract was divided into 50 doses in 5 mL of normal saline and stored at a temperature of -70 °C. All subjects were fed with antigen extracted from their own intestinal mucosa. A 2-wk supply was given to the subject who at each visit was instructed to maintain the vials in a refrigerator at home. Each subject ingested 3 doses per week for 16 wk, a total of 48 doses. Two of the five milliliters of vial doses were kept as retained.
Clinical and laboratory follow-up Study individuals were monitored with a variety of safety, biologic and efficacy parameters during the baseline, feeding, and post-feeding periods. The safety parameters included general clinical safety parameters to monitor the individual’s overall status as well as specific organ systems, and antigen-specific parameters relevant to the administration of the study drugs.
Safety and tolerability of oral administration of the study drugs were determined by evaluation of clinical parameters as detailed by the subject in his/her diary entries; by biweekly physical examinations and medical history, and biweekly laboratory evaluations. A designated physician involved in the study was accountable for interim history, vital signs, body weight, adverse event assessment and physical examination. Complete blood counts (CBC), sedimentation rate (ESR) and standard chemistries (SMA) were performed every 2 wk throughout the study. Evaluation of the effect of oral administration of the study drug on amelioration of the immune-mediated intestinal and extraintestinal injury in subjects with CD was assessed by following the biweekly CDAI score. IBDQ was evaluated at baseline and at the end of treatment period (16 wk). Response was determined if any of the following occurred: a decrease in CDAI score from time 0 by 70 points, or a decrease to a level ≤150 points, which defines clinical remission.
Evaluation of the ability to induce immune regulation by oral administration of the study drug was assessed by monthly serum cytokine levels, and by analysis of T-cell subpopulations biweekly, as described below. IFNγ ELISPOT assay was performed at wk 0 and 32.
Flow cytometry analysis for determination of the effect of oral immune regulation on CD4, CD8, NKT, CD3+/45RA, CD3+/45RO, CD19+/45RA, CD25+/45RO, CD4+/45RO, CD8+/45RO, CD44+/45RO, and CD16+/56+ lymphocytes in peripheral blood Blood samples were collected throughout the study period. Immediately after lymphocyte isolation, duplicates of 2-5×104 cells/500 μL PBS were deposited into Falcon 2052 tubes incubated with 4 mL of 1% BSA for 10 min, and centrifuged at 1400 r/min for 5 min. Cells were resuspended in 10 μL FCS with 1:20 FITC-antihuman CD4, CD8, NKT, CD3+/45RA, CD19+/45RA, CD25+/45RO, CD3+/45RO, CD4+/45RO, CD8+/45RO, CD44+/45RO, or CD16+/56+ antibodies (Pharmingen, and R&D, USA), and mixed every 10 min for 30 min. Cells were washed twice in 1% BSA, 0.5 mL 1% paraformaldehyde was added, and kept at 4 °C until reading. For the control group, only 5 μL of 1% BSA was added. Analytical cell sorting was performed on 1×104 cells from each group with a fluorescence-activated cell sorter (FACSTAR plus, Becton Dickinson). Only live cells were counted and background fluorescence from non-antibody-treated lymphocytes was deducted from levels obtained.
IFNγ ELISPOT assays IFNγ spot forming cells (SFC) were determined using a colitis extracted protein (CEP)-specific ELISPOT assay (Mabtech, Nacka, Sweden) as described with the following modifications[27]. In brief, 96-well filtration plates coated with high protein binding hydrophobic PVDF membrane (polyvinylidene disulfide) were used (Millipore Corp., Bedford, MA, USA). Plates were coated with 1-D1K anti-IFNγ coating antibody (15 mg/mL, Mabtech) for 24 h at 4 °C. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient separation from 20 mL blood samples, collected in acid citrate dextrose tubes, and processed within 1 h. PBMCs were washed twice in RPMI 1640 with 10% fetal calf serum. Cells were cultured in 96-well plates (1×105 cells/well) with RPMI 1640 and 10% FCS. Three triplicates were prepared with CEP from each patient (50 μg/mL), phytohemagglutinin (PHA, 2.5 μg/mL), or RPMI without antigen. Plates were incubated for 48 h at 37 °C and 50 mL/L CO2. Following washing, dilute biotinylated antibodies (7-B6-1-biotin, Mabtech) were added in filtered PBS with 0.5% FCS to 1 μg/mL, in a total volume of 100 μL/well. Plates were incubated for 3 h at room temperature. Following washing, 100 mL of streptavidin-alkaline phosphatase was added, and plates were incubated for 90 min at room temperature. After washing, a substrate was added (BCIP/NBT, BioRad, Richmond, USA) for 30 min until dark red purple spots emerged. Using a dissection microscope, two independent investigators counted dark spots, reflecting IFNγ-secreting clones. Results are expressed as means of triplicate IFNγ-secreting cells per 105 PBMCs, after subtracting the mean spots from wells without viral antigens.
IFNγ and IL-4 serum levels IL-4, IL-10, and IFNγ serum levels were measured by a “sandwich” ELISA, using Genzyme Diagnostics kits (Genzyme Diagnostics, MA, USA) according to the manufacturer’s instructions.
Statistical analysis
Summary statistics by time point of all clinical and laboratory variables were calculated, and statistical significance of changes from baseline was assessed by Student’s t-test at each time point.
RESULTS
Patient characteristics
One group of 10 patients was studied. They included six males and four females with a mean age of 37 years (range 19-59 years). Mean duration of disease was 11 years (range 2-37 years). Disease site was the ileum in six patients, the ileocolon in two patients, and the colon in two patients. Two patients underwent prior intestinal resection; two suffered from fistulae. Concomitant medications: Five patients were treated with 5-ASA and five were treated with corticosteroids. Steroid treatment was discontinued in two patients and significantly reduced in one patient throughout the study period.
Effect of oral immune regulation towards autologous colitis extracted proteins on CDAI score
Administration of the study drug significantly ameliorated disease activity. During the course of treatment, 10/10 subjects had a clinical response (CDAI decrease of at least 70 points), and 7/10 subjects achieved clinical remission (CDAI ≤ 150). Median time to response was 5 wk (Figures 1A and 1B). The median decrease of CDAI was -102 points in 6 wk (from 264 to 162 points, P = 0.003), and -129 points in 14 wk (135 points, P = 0.0001). At the end of the non-treatment 16-wk follow-up period, CDAI score increased to 206 points.
Figure 1.
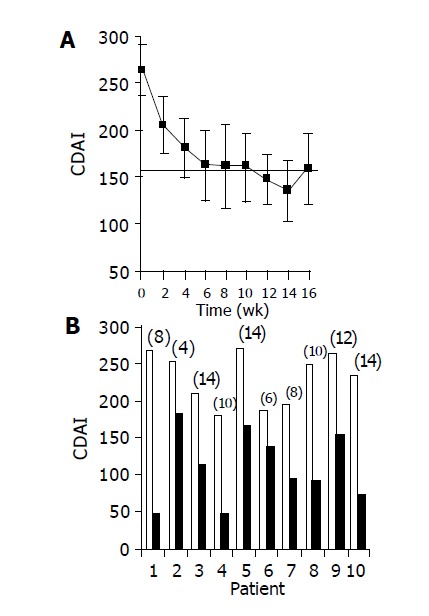
Effect of the study drug on CDAI score. A: mean±SE CDAI scores over time during the treatment period; B: CDAI scores of individual patients: Baseline score (wk 0, open bars) as compared with score at time of maximal decrease (black bars, no. of week appears for each patient in parenthesis above bar).
Effect of oral immune regulation towards autologous colitis extracted proteins on IBDQ score
Administration of the study drug significantly ameliorated disease activity as measured by IBDQ score. A significant increase in mean IBDQ score was noted at wk 16 as compared to baseline (134±9 vs 164±12, P<0.05, Figures 2A and 2B).
Figure 2.
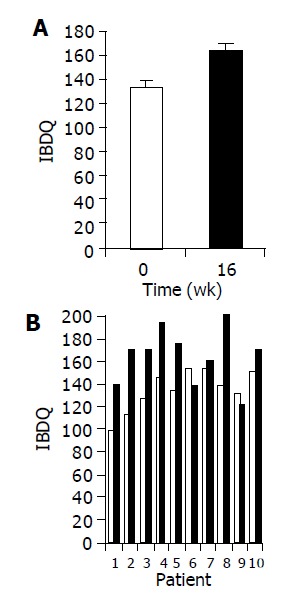
Effect of the study drug on IBDQ scores. A: Mean IBDQ scores (±SE) at baseline (wk 0, open bars) as compared to end of treatment (wk 16, black bars); B: IBDQ score of individual patients at baseline as compared to wk 16.
Effect of oral immune regulation towards autologous colitis extracted proteins on subpopulations of T lymphocytes
Administration of the study drug significantly induced a significant increase in the CD4+/CD8+ lymphocyte ratio, in 7/10 subjects (Figure 3). The peripheral NKT cell number increased significantly in 5/10 subjects (Figure 4). A significant increase in the CD3+/45RA (Figure 5), CD19+/45RA (Figure 6), and CD25+/45RO (Figure 7) lymphocytes was noted in the majority of treated subjects (Figure 5). No major changes occurred in CD3+/45RO, CD4+/45RO, CD8+/45RO, CD44+/45RO, and CD16+/56+ lymphocytes subpopulations.
Figure 3.
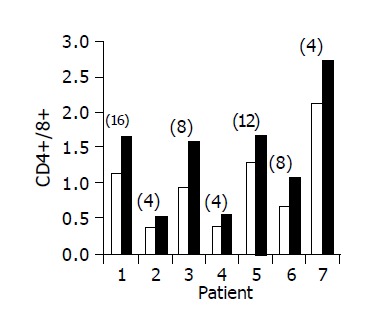
Peripheral CD4+/CD8+ lymphocyte ratio in 7/10 patients in whom a significant increase was observed. Baseline ratio (open bars) as compared to level of maximal increase (black bars, no. of week shown in parenthesis above each bar).
Figure 4.
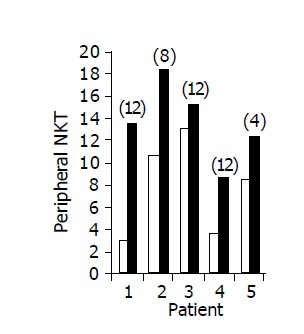
Peripheral NKT lymphocyte percent in 5/10 patients in whom a significant increase was observed during treatment. Baseline ratio (open bars) as compared to level of maximal increase (black bars, no. of week shown in parenthesis above each bar).
Figure 5.
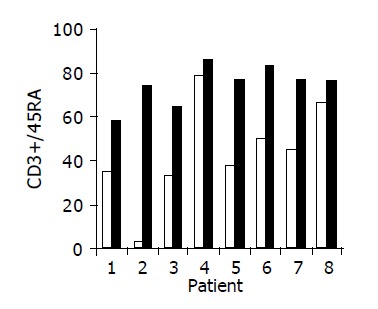
Peripheral CD3+/45RA lymphocyte ratio in 8/10 patients in whom a significant increase was observed during treatment. Baseline ratio (open bars) as compared to level of maximal increase (black bars).
Figure 6.
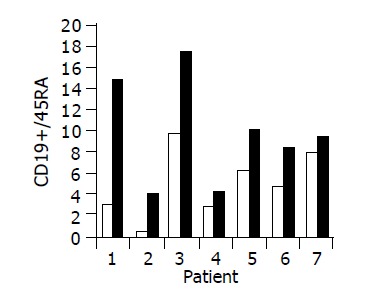
Peripheral CD19+/45RA lymphocyte ratio in 7/10 patients in whom a significant increase was observed during treatment. Baseline ratio (open bars) as compared to level of maximal increase (black bars).
Figure 7.
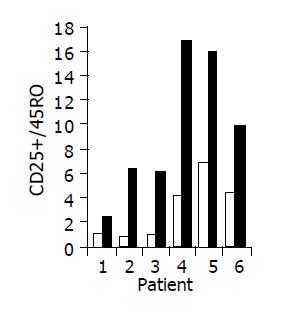
Peripheral CD25+/45RO lymphocyte ratio in 6/10 patients in whom a significant increase was observed during treatment. Baseline ratio (open bars) as compared to level of maximal increase (black bars).
Effect of oral immune regulation towards autologous colitis extracted proteins on antigen specific IFNγ-producing T cell clones
The antigen-specific effect of oral immune regulation on IFNγ SFC was determined using a CEP-specific ELISPOT assay. In five patients, positive T-cell clones were detected prior to oral protein administration. In all five, a significant decrease in the number of IFN-positive SFC was noted (0.2-3.6 to 0-0.2 SFC).
Effect of oral immune regulation towards autologous colitis extracted proteins on serum cytokine levels
A significant increase in IL-4 (from 0.17±0.1 to 0.63±0.15 pg/mL, P<0.05), and IL-10 (from 1.7±0.61 to 3.5±1.02 pg/mL, P<0.05) serum levels was observed in 7/10 subjects during treatment period (Figures 8 and 9). No significant changes in serum IFNγ levels were observed.
Figure 8.
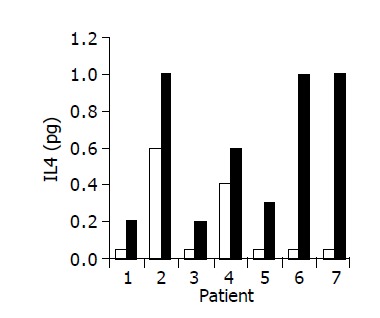
Effect of the study drug on serum IL-4 levels in 7/10 patients in whom a significant increase was observed during treatment. Baseline ratio (open bars) as compared to level of maximal increase (black bars).
Figure 9.
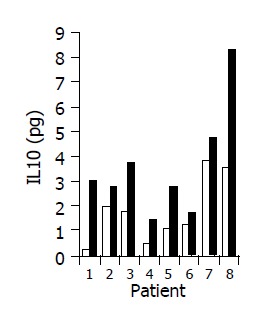
Effect of the study drug on serum IL-10 levels in 8/10 patients in whom a significant increase was observed during treatment. Baseline ratio (open bars) as compared to level of maximal increase (black bars).
Safety measures
Treatment was well tolerated by all patients. All 10 patients completed the study and no major treatment-related adverse reactions were noted. A physician followed up the interim history, vital signs, body weight, adverse event assessment and performed a physical examination. CBC, ESR and standard chemistries (SMA) were performed every 2 wk throughout the study. No major treatment-related adverse events were reported or observed in any of the treated patients during the feeding and follow-up periods. No major changes in any of the extraintestinal systems monitored were reported in any of the patients.
DISCUSSION
The results of the present study suggest that induction of oral immune regulation via oral administration of an autologous colon-specific protein-containing extract is a safe and could possibly be an effective treatment for subjects with mild to moderate CD. Induction of oral immune regulation towards CEP appeared to significantly ameliorate disease activity. During the course of treatment period, 10/10 subjects had a favorable clinical response (CDAI≤70), and 7/10 subjects achieved clinical remission (CDAI≤150). Median time to response was 5 wk. A significant increase in mean IBDQ score was noted at wk 16 as compared to baseline. No treatment-related adverse events were noted in any of the subjects.
The data of the present study provide a clue to the mechanism associated with the clinical effects. High levels of CEP-specific IFNγ spot forming colonies were detected in five subjects prior to treatment. In all five, a marked decrease was observed. A significant increase in IL-10 and IL-4 serum levels was observed in 7/10 subjects during the treatment period. These results suggest that immune regulation by the study drug could alter bowel-associated antigen-specific immunity, and may induce a systemic Th1 to Th2 immune shift.
Successful treatment of IBD, similar to that of other immune-mediated disorders, requires mitigation of the immune response towards disease-associated target antigens. This involves generalized immunosuppression, which may bring undesirable side effects[1,12]. Several new immunomodulatory treatments were tested in patients with IBD over the last few years. These treatments are all non-antigen specific[12]. This lack of disease-associated antigens’ specificity may explain the relatively low response rate, as well as the relatively high levels of unwanted side effects. In contrary, induction of antigen-specific oral immune regulation towards autologous colon specific protein-containing extracts could potentially enable long-term alleviation of the disease, while leaving the general immunological defense of the recipient intact[9,22-26]. In addition, as all of the new immune modulatory agents involve systemic immune suppression, their long-term effects are yet to be tested. On the contrary, antigen-specific oral immune regulation is unlikely to have long-term effects in other systems.
Induction of oral immune regulation has been used to alleviate several immune-mediated disorders in animals, including collagen-induced arthritis, experimental colitis, chronic graft vs host disease, and experimental encephalomyelitis[13-20]. We have previously shown that oral immune regulation towards adenoviral antigens can abrogate the humoral and cellular components of the anti-viral immunity[18,27]. It was also shown that oral administration of low-dose HBV-envelope proteins (BioHepB) induced peripheral humoral immune tolerance towards HBV epitopes in naive animals. In addition, tolerance induction downregulated any pre-existing anti-HBV immune response, and inhibited anti-HBs antibody production in mice with secondary anti-viral immunity[27]. This mode of treatment was tested in patients with rheumatoid arthritis, autoimmune uveitis, type I diabetes mellitus, and multiple sclerosis[21,28-31]. We have recently shown that this method is effective in patients with chronic HBV infection[32]. The results of these studies showed that HBV-specific T-cell immune modulation can be elicited via oral administration of HBV envelope proteins to chronically infected individuals. In the experimental colitis model, induction of oral immune regulation was shown by several groups to alleviate the disease[22]. This response was associated with a reverse of the cytokine secretion paradigm with increased secretion of IL-4 and decreased secretion of IFNγ. Adoptive transfer of tolerance by transplantation of immune cells from orally-tolerized donors to sublethally irradiated recipients supports the existence of suppressor cells in this setting[22].
As the intestinal damage in CD patients results mostly from the host immune response, two explanations may elucidate these results, the first being the principle of induction of immune tolerization. Oral immune regulation towards colitis proteins may have altered the immune deviation, thereby removing a deleterious T-cell population (such as those that secrete IFNγ), thus uncovering a more efficacious sub-dominant response (secreting anti-inflammatory cytokines such as IL-4 and IL-10). The second possible explanation is induction of immunity. Oral immune regulation may have enhanced the effect of a beneficial subset of T cells towards the fed antigens in these patients, or of a regulatory subtype of T lymphocytes that reintroduce the required immunological balance in this setting. Correction of an immunological imbalance can lead to an enhanced effect on the part of T-cell subtypes, rather than a clearance or irreversible suppression of “unwanted” T cells. It is possible that simultaneous downregulation of one subset of T cells and augmentation of another occurs. Similar approaches were recently described towards Schistosoma mansoni and HBV infections, in which the immune response was responsible for the disease[32,33]. Inherent in this concept is the understanding that pathology is not essential for the development of a protective response. As it is not always possible to separate pathology from “excessive” immune protection, it might not always be possible to determine the role of different antigens and/or subsets of T cells in the induction of each response.
The data of the present study showed a significant increase in the peripheral CD4+/CD8+ lymphocyte ratio in 7/10 patients, and of the peripheral NKT cell numbers in 5/10 subjects. Interestingly, in the experimental colitis model, tolerance induction led to a significant increase in NKT numbers[26,34-36]. The peripheral CD4+/CD8+ ratio increased threefold in tolerized vs non-tolerized mice. An opposite effect was observed in the intrahepatic CD4+/CD8+. T lymphocytes expressing NK cell markers (NKT cells) exist in low numbers in the peripheral blood and most other tissues, but are abundant in the liver and bone marrow[37]. They are typified as CD4+ or CD4-CD8- and CD16-, and express αβ TCRint. Upon in vivo and in vitro stimulations, they produce large amounts of both IL-4 and IFNγ, exhibit enhanced cytolytic activity, and have a regulatory role in helper T (Th) cell differentiation[38-40]. NKT lymphocytes were shown to play a regulatory role in immune-mediated disorders. Others and we have recently shown that this subset of lymphocytes may have a role in peripheral tolerance induction. Induction of peripheral tolerance via oral administration of an antigen, or FK506 treatment, was associated with significant increases in intrahepatic NKT lymphocyte proportions and cytotoxicity function[36]. Depletion of NKT lymphocytes prevented the Th1 to Th2 immune shift, hindering the ability to induce immune tolerance in experimental colitis[26]. The results of the present study suggest that oral immune regulation induces a systemic immune shift in subpopulations of T cells. However, based on the present data it is difficult to draw conclusions on the exact role of subtypes of T lymphocytes in the pathogenesis of CD, as well as on the regulatory role of NKT cells in this setting.
Application of oral tolerance in some patients with IBD may require the use of surrogate antigens. A similar approach has been used in trials involving patients with multiple sclerosis, diabetes, and rheumatoid arthritis[41-45]. A bystander effect is known to play a role in oral immune regulation[44]. It involves regulatory cells secreting non-antigen-specific cytokines that suppress inflammation in the microenvironment, where the fed antigen is localized. Mucosal Th2/Th3 cells, secreting TGFβ generated by intermittent feeding of a low-dose antigen, may play a role in bystander tolerance[13]. We have previously shown that surrogate antigens derived from normal colonic wall and those derived from other species induced a beneficial effect in experimental colitis[35]. These results suggest that surrogate antigens, related to the disease-target epitopes, may have a similar immune modulating effect. They imply that closely related proteins are being presented and processed by gut-associated lymphoid tissue in a similar way. Both administration of an antigenitically similar epitope, or of an epitope distinct from the disease-target antigen but found in the target organ, can regulate peripheral immune activation. Geographical proximity and/or antigenic similarity to the disease-target antigen may be held responsible for these effects. In the present study, biopsies were taken preferentially from inflamed areas in the colon, or the terminal ileum. In some of the patients, however, biopsies were taken from mucosa of a normal appearance. In both, a clinical effect was noted.
During the periods of follow-up, 5 of the 10 patients experienced a relapse of their symptoms. This loss of effect may be due to the partial effect of oral immune regulation on subtypes of regulatory T lymphocytes in this setting. It may suggest that long-term treatment, and even continuous exposure of the disease-associated antigens, are required in these settings.
In summary, induction of oral immune regulation towards colitis-extracted proteins is a safe and perhaps effective new mode of therapy in patients with mild to moderate CD. This novel method significantly alleviated the disease while altering the antigen-specific immune response. It had no undesirable effects in other systems. As the present study was an open non-randomized trial, large-scale double blind placebo trials would be required in order to evaluate this new mode of treatment for CD patients.
Footnotes
Supported by the Enzo Therapeutics Inc., NY, USA
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Su C, Lichtenstein GR. Recent developments in inflammatory bowel disease. Med Clin North Am. 2002;86:1497–1523. doi: 10.1016/s0025-7125(02)00085-8. [DOI] [PubMed] [Google Scholar]
- 3.Rimm DL, Holland TE, Morrow JS, Anderson JM. Autoantibodies specific for villin found in patients with colon cancer and other colitides. Dig Dis Sci. 1995;40:389–395. doi: 10.1007/BF02065426. [DOI] [PubMed] [Google Scholar]
- 4.Hibi T, Aiso S, Ishikawa M, Watanabe M, Yoshida T, Kobayashi K, Asakura H, Tsuru S, Tsuchiya M. Circulating antibodies to the surface antigens on colon epithelial cells in ulcerative colitis. Clin Exp Immunol. 1983;54:163–168. [PMC free article] [PubMed] [Google Scholar]
- 5.Das KM, Vecchi M, Sakamaki S. A shared and unique epitope(s) on human colon, skin, and biliary epithelium detected by a monoclonal antibody. Gastroenterology. 1990;98:464–469. doi: 10.1016/0016-5085(90)90839-s. [DOI] [PubMed] [Google Scholar]
- 6.Strober W, Nourish MF. Immunological diseases of the gastrointestinal tract. In: RR Rich, Ed , editors. Clinical Immunology. St. Louis: Mosey; 1995. pp. 1401–1428. [Google Scholar]
- 7.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 9.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 11.van Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997;113:383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]
- 12.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 13.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 14.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 15.Ilan Y. Immune downregulation leads to upregulation of an antiviral response: a lesson from the hepatitis B virus. Microbes Infect. 2002;4:1317–1326. doi: 10.1016/s1286-4579(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 16.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Herrath MG, Dyrberg T, Oldstone MB. Oral insulin treatment suppresses virus-induced antigen-specific destruction of beta cells and prevents autoimmune diabetes in transgenic mice. J Clin Invest. 1996;98:1324–1331. doi: 10.1172/JCI118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilan Y, Prakash R, Davidson A, Jona G, Horwitz MS, Chowdhury NR, Chowdhury JR. Oral tolerization to adenoviral antigens permits long-term gene expression using recombinant adenoviral vectors. J Clin Invest. 1997;99:1098–1106. doi: 10.1172/JCI119238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagler A, Pines M, Abadi U, Pappo O, Zeira M, Rabbani E, Engelhardt D, Ohana M, Chowdhury NR, Chowdhury JR, et al. Oral tolerization ameliorates liver disorders associated with chronic graft versus host disease in mice. Hepatology. 2000;31:641–648. doi: 10.1002/hep.510310314. [DOI] [PubMed] [Google Scholar]
- 20.Ilan Y, Gotsman I, Pines M, Beinart R, Zeira M, Ohana M, Rabbani E, Engelhardt D, Nagler A. Induction of oral tolerance in splenocyte recipients toward pretransplant antigens ameliorates chronic graft versus host disease in a murine model. Blood. 2000;95:3613–3619. [PubMed] [Google Scholar]
- 21.Garside P, Mowat AM. Oral tolerance. Semin Immunol. 2001;13:177–185. doi: 10.1006/smim.2001.0310. [DOI] [PubMed] [Google Scholar]
- 22.Ilan Y, Weksler-Zangen S, Ben-Horin S, Diment J, Sauter B, Rabbani E, Engelhardt D, Chowdhury NR, Chowdhury JR, Goldin E. Treatment of experimental colitis by oral tolerance induction: a central role for suppressor lymphocytes. Am J Gastroenterol. 2000;95:966–973. doi: 10.1111/j.1572-0241.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 23.Galliaerde V, Desvignes C, Peyron E, Kaiserlian D. Oral tolerance to haptens: intestinal epithelial cells from 2,4-dinitrochlorobenzene-fed mice inhibit hapten-specific T cell activation in vitro. Eur J Immunol. 1995;25:1385–1390. doi: 10.1002/eji.1830250537. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta A, Ramaswamy K, Giraldo J, Taniguchi M, Amenta PS, Das KM. Colon epithelial cellular protein induces oral tolerance in the experimental model of colitis by trinitrobenzene sulfonic acid. J Lab Clin Med. 2001;138:257–269. doi: 10.1067/mlc.2001.118221. [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta A, Kesari KV, Ramaswamy KK, Amenta PS, Das KM. Oral administration of unmodified colonic but not small intestinal antigens protects rats from hapten-induced colitis. Clin Exp Immunol. 2001;125:41–47. doi: 10.1046/j.1365-2249.2001.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trop S, Samsonov D, Gotsman I, Alper R, Diment J, Ilan Y. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 1999;29:746–755. doi: 10.1002/hep.510290334. [DOI] [PubMed] [Google Scholar]
- 27.Gotsman I, Beinart R, Alper R, Rabbani E, Engelhardt D, Ilan Y. Induction of oral tolerance towards hepatitis B envelope antigens in a murine model. Antiviral Res. 2000;48:17–26. doi: 10.1016/s0166-3542(00)00113-3. [DOI] [PubMed] [Google Scholar]
- 28.Weiner HL, Friedman A, Miller A, Khoury SJ, al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 29.Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, Hafler DA. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 30.Trentham DE. Oral tolerization as a treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 1998;24:525–536. doi: 10.1016/s0889-857x(05)70024-7. [DOI] [PubMed] [Google Scholar]
- 31.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson CO. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol. 1994;152:4663–4670. [PubMed] [Google Scholar]
- 32.Safadi R, Israeli E, Papo O, Shibolet O, Melhem A, Bloch A, Rowe M, Alper R, Klein A, Hemed N, et al. Treatment of chronic hepatitis B virus infection via oral immune regulation toward hepatitis B virus proteins. Am J Gastroenterol. 2003;98:2505–2515. doi: 10.1111/j.1572-0241.2003.07700.x. [DOI] [PubMed] [Google Scholar]
- 33.McSorley SJ, Garside P. Vaccination by inducing oral tolerance? Immunol Today. 1999;20:555–560. doi: 10.1016/s0167-5699(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 34.Gotsman I, Shlomai A, Alper R, Rabbani E, Engelhardt D, Ilan Y. Amelioration of immune-mediated experimental colitis: tolerance induction in the presence of preexisting immunity and surrogate antigen bystander effect. J Pharmacol Exp Ther. 2001;297:926–932. [PubMed] [Google Scholar]
- 35.Shlomai A, Trop S, Gotsman I, Jurim O, Diment J, Alper R, Rabbani E, Engelhardt D, Ilan Y. Immunomodulation of experimental colitis: the role of NK1.1 liver lymphocytes and surrogate antigens--bystander effect. J Pathol. 2001;195:498–507. doi: 10.1002/path.974. [DOI] [PubMed] [Google Scholar]
- 36.Samsonov D, Trop S, Alper R, Diment J, Ilan Y. Enhancement of immune tolerance via induction of NK1.1 positive liver-associated-lymphocytes under immunosuppressive conditions. J Hepatol. 2000;32:812–820. doi: 10.1016/s0168-8278(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald HR. NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 39.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 40.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–149. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZY, Lee CS, Lider O, Weiner HL. Suppression of adjuvant arthritis in Lewis rats by oral administration of type II collagen. J Immunol. 1990;145:2489–2493. [PubMed] [Google Scholar]
- 42.von Herrath MG. Bystander suppression induced by oral tolerance. Res Immunol. 1997;148:541–554. doi: 10.1016/s0923-2494(98)80148-x. [DOI] [PubMed] [Google Scholar]
- 43.Karpus WJ, Kennedy KJ, Smith WS, Miller SD. Inhibition of relapsing experimental autoimmune encephalomyelitis in SJL mice by feeding the immunodominant PLP139-151 peptide. J Neurosci Res. 1996;45:410–423. doi: 10.1002/(SICI)1097-4547(19960815)45:4<410::AID-JNR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundin BS, Dahlgren UI, Hanson LA, Telemo E. Oral tolerization leads to active suppression and bystander tolerance in adult rats while anergy dominates in young rats. Scand J Immunol. 1996;43:56–63. doi: 10.1046/j.1365-3083.1996.d01-15.x. [DOI] [PubMed] [Google Scholar]


