Abstract
AIM: Esomeprazole, an oral S-form of omeprazole, has been a greater acid inhibitor over omeprazole in treating acid-related diseases. Only less published data is available to confirm its efficacy for Asian people. Therefore, a perspective, double-blind, randomized comparison of esomeprazole tablets 40 mg (Nexium®) vs omeprazole capsules 20 mg (Losec®) in treating Chinese subjects with erosive/ulcerative reflux esophagitis (EE) was conducted.
METHODS: A total of 48 EE patients were enrolled and randomized into two treatment groups under 8-wk therapy: 25 receiving esomeprazole, while another 23 receiving omeprazole treatment. Finally, 44 completed the whole 8-wk therapy.
RESULTS: The difference in healing EE between two groups was 22.7% (72.7% vs 50.0%), not reaching significant value (P = 0.204). The median of the first time needed in relieving heartburn sensation was 1 d for both groups and the remission rates for heartburn on the 1st d after treatment were 77.3% and 65%, respectively (NS). The scores of various reflux relieving symptoms evaluated either by patients or by investigators were not different. Regarding drug safety, 28% of esomeprazole group and 26.1% of omeprazole group reported at least one episode of adverse effects, while constipation and skin dryness were the common side effects in both groups (NS).
CONCLUSION: Esomeprazole 40 mg is an effective and safe drug at least comparable to omeprazole in treating Chinese EE patients.
Keywords: Esomeprazole, Someprazole, Esophagitis
INTRODUCTION
The reflux of gastric acid and duodenal contents into esophagus is a normal physiological phenomenon. However, the sustained esophageal mucosal damage, e.g., erosive/reflux esophagitis induced by this kind of reflux, may happen when the normal esophageal clearance and mucosal protection ability are impaired[1]. Gastroesophageal reflux disease (GERD) refers to individuals who are exposed to the physical complications from this reflux, or who experience clinically significant impairment of healthy well-being and quality of life due to reflux-related symptoms[2]. Today, prompt and effective relief of GERD symptoms is the primary goal for these patients. Since acid has been the major pathogen leading to reflux-associated symptoms, current GERD treatment is mainly aimed to reduce the acid exposure to esophagus[3,4]. For example, omeprazole (Losec®), the first proton pump inhibitor (PPI) showing an effective acid inhibitory ability, provides the satisfactory therapy either in GERD symptom relief or in healing of erosive esophagitis[5-7]. The modified formulation of omeprazole, multiple unit pellet system, remains effective in healing and relieving symptoms in GERD patients[8]. Up to date, omeprazole efficacy and safety are well established in many trials because more than 600 million patients have used omeprazole capsules worldwide including Taiwan[9,10].
Esomeprazole (Nexium®), the new S-isomer of omep-razole, is introduced to reduce gastric acid secretion more efficiently[9]. Unlike omeprazole, pharmacodynamic data suggest that the metabolism of esomeprazole in human liver microsomes is less dependent on CYP2C19 but mainly via CYP3A4[11]. Based on this observation, perhaps the inter-individual variation of esomeprazole metabolism is less compared to omeprazole[12]. In addition, studies have pointed out that esomeprazole exhibits significantly higher bioavailability, leading to the greater inhibition of gastric acid secretion compared to omeprazole[11,13]. Accordingly, many studies conducted in Western countries have confirmed the superior efficacy of esomeprazole over omeprazole in treating GERD patients[9,14].
GERD appears less common in East Asian countries compared to Western ones[15,16]. It is of interest to know the reason for the efficacy of esomeprazole in Asian GERD patients. Kao et al[17], indicated that esomeprazole achieved 68-73.9% sustained symptomatic response rate for GERD patients in an on-demand therapy trial. Based on the study design of a double-blind, randomized and controlled trial, the purpose of our study was to compare the efficacy and safety of esomeprazole tablet 40 mg and omeprazole capsule 20 mg in treating patients with endoscopically confirmed reflux esophagitis (EE) enrolled in a single center. Our primary objective was to assess the EE healing rate using both agents by an 8-wk treatment period. While the secondary objectives were to compare the response of reflux symptoms and general well-being by both agents at wk 4 and 8, respectively, to compare the time needed to relieve heartburn by both agents, and to evaluate the tolerability and safety of both agents.
MATERIALS AND METHODS
Design and study population
This was an active-controlled, double-blinded, double-dummy, randomized, single-center study with a parallel group designed to enroll 48 EE patients. Forty-eight outpatients (M/F: 38/10, age: 54.1±17.8 years), who sought medical care because of typical GERD symptoms for at least 1 mo, were consecutively enrolled in the study. They all received an endoscopy to confirm the EE diagnosis according to Los Angeles (LA) grading system[18], and met an inclusion criterion for an 8-wk treatment period with either esomeprazole tablet 40 mg or omeprazole capsule 20 mg (AstraZeneca, Gothenburg, Sweden). While endoscopy specimens were simultaneously obtained from stomach antrum and body for a rapid urease test to determine Helicobacter pylori (H pylori) infection. Those subjects with the following conditions were excluded: coexistence of healed or active peptic ulcer, gastrointestinal malignancy, esophago-gastric surgical history, esophagitis obviously resulted from systemic diseases, infections, drugs, burn, radiotherapy or physical deformity, severe esophageal stricture requiring dilatation at first endoscopy or expectation of requiring dilatation during the study, recent PPI treatment within 8 d prior to endoscopy, or using PPIs for more than 5 d in the last 28 d prior to endoscopy, with H pylori eradication therapy within the last 28 d prior to randomization or at any time during the study, using other antisecretory or prokinetic agents between endoscopy and randomization or at any time during the study, or with any investigational (non-approved) drug during the last 30 d prior to randomization. In addition, those with severe concurrent diseases judged by the investigators to complicate the evaluation of the trial, pregnancy, lactation or child-bearing potential without adequate contraception (contraceptive pill or intrauterine device), chronic alcoholism, drug abuse or any other conditions associated with poor patient compliance including expected non-co-operation, previous randomization in the study, and patients who needed continuously concomitant therapy with anticholinergics, cisapride, prostaglandin analogs, non-steroidal anti-inflammatory drugs (including COX-II) and aspirin (excluding low-dose aspirin e.g., 100 mg, as anti-platelet) were also excluded from this study. This study was approved by the Ethical Committee of Taipei Veterans General Hospital and carried out in accordance with the World Medical Association Helsinki Declaration. Written informed consent was obtained before any study-related procedures were performed.
The GERD symptoms such as heartburn, acid regurgitation, epigastric/chest pain, belching, nausea, vomiting and global well-being were assessed based on a standard visual analog scaled (VAS) questionnaire. Study medication was administered only to those subjects included in this study, following the procedures set out in the clinical study protocol. A sequence of patient numbers was assigned to the study center. All subjects entering the study received a patient number. This patient number was printed on the case report form and was used to identify the subject throughout the study. A randomization schedule was generated by the AstraZeneca using a validated system that automated the random assignment of treatment groups according to the randomization numbers. This schedule linked sequential numbers to treatment codes allocated at random. The schedule was prepared with a 1:1 randomization ratio in block size of 4. The study medication was labeled with the randomization numbers (medication numbers). At the end of baseline visit, eligible patients were randomized to the study medication in accordance with the randomization schedule. The next eligible subject received the study medication with the lowest available randomization number. Each subject was given only the study medication carrying his/her randomization number. The investigator documented the randomization number by sticking the label provided on the appropriate case report form. Subjects who permanently discontinued from the study were to retain their subject number and their randomization number, if already given. New subjects were always allotted a new subject number and, if applicable, a new randomization number.
Patients were asked to come back to the study office on three occasions (baseline, wk 4 and 8, respectively) during the trial. All doses were taken by mouth once daily in the morning before breakfast. During the study period, the patients were instructed to take one tablet of esomeprazole or matching placebo and one capsule of omeprazole or matching placebo in the morning with a glass of water. The first dose of study medication was taken on the morning after randomization. This was considered as d 1 of treatment. After the whole course of treatment, they were asked to come back on the last day of medication. At that time, they received the 2nd endoscopy to assess the EE status again. Meanwhile GERD symptoms based on VAS after treatment were scored again. All the endoscopic EE diagnoses and their follow-up according to LA grading were initially performed by an experienced endoscopist (Chang), while all the endoscopic findings including EE were recorded by Polaroid films. When all the studies were completed, the recorded films assigned to their study codes were reviewed independently by another two endoscopists (Lu and Chen) who were blind to the order and code of endoscopy for each patient. If three readings in each film were dissimilar, this film was discussed by the above three investigators together to obtain the final endoscopic assessment. Healing was defined as no EE evidence.
Efficacy data
The primary endpoint of this trial was the percentage of enrolled patients whose EE was healed by wk-8 visit. The secondary efficacy variables included for the first time the relief of heartburn symptoms, changes of reflux symptom scores (based on VAS score) and overall therapeutic effects judged via either subjective (patient) or objective (investigator) assessment.
Safety data
The safety assessments included observed and reported adverse events (AE) and clinical laboratory evaluations (hematology and serum chemistry).
Study duration and dates
The study took place between 28 March, 2001 and 26 October, 2001.
Statistical procedures
For the primary efficacy parameter, the healing rate for each group was calculated with 95%CI. The difference between the two treatment groups and the corresponding 95%CI were also provided and compared by using Fisher’s exact test. The cumulative percentage of patients who exhibited the first relief of their diary-recorded symptom of heartburn was compared using Fisher’s exact test on d 1, 7, and 28, respectively. The median first time to relieve heartburn symptom between the two groups were compared using log-rank test. For reflux symptoms, Wilcoxon rank sum test was used to assess patient VAS score, and Fisher’s exact test was used to assess investigator scores as well as the overall therapeutic effect.
AE were summarized according to coding symbols for thesaurus of adverse reaction terms. The number of patients who reported a particular event and the number of events were summarized. Comparative incidence of AE was evaluated using Fisher’s exact test. Each laboratory parameter was listed with values outside the reference range identified. For each laboratory parameter, changes in abnormality/normality status from pre- to post-treatment were summarized in shift tables and assessed using McNemar test.
Interim analysis
No interim analysis was performed in this study.
RESULTS
Study subjects and conduct
Finally, 48 eligible EE patients were enrolled and randomized according to the protocol. Their demographics and baseline characteristics are summarized in Table 1. Of them, 25 patients were distributed into esomeprazole group whereas 23 patients were on omeprazole treatment. Table 1 illustrates that both groups were comparable in their demographic characteristics and basal clinical manifestations except the subjects of esomeprazole group had a higher chance of belching (P<0.05). Among the 48 randomized patients, only 44 (esomeprazole: 24; omeprazole: 20) completed the whole study course. The reasons why four of them did not finish the trial were as follows: two lost their follow-up and another two discontinued the study medication. In addition, 2 (all were esomeprazole) of 44 who finished the study refused endoscopy follow-up at wk-8 visit were excluded from per protocol analysis.
Table 1.
Demographics and baseline characteristics of erosive esophagitis patients treated with esomeprazole or omeprazole (mean±SE).
| Esomeprazole 40 mg n = 25 | Omeprazole 20 mg n = 23 | P | |
| Age (yr) | 49.2±3.7 | 59.0±3.4 | 0.0596 |
| Sex (male%) | 20 (80) | 18 (78.3) | 1.0000 |
| Body weight (kg) | 68.4±2.4 | 70.9±2.5 | 0.4779 |
| Height (cm) | 166.7±1.3 | 169.0±1.4 | 0.2096 |
| Basal reflux symptoms (VAS) | |||
| Heartburn | 29.4±5.7 | 23.6±5.9 | 0.2683 |
| Nausea | 18.7±5.5 | 13.8±5.8 | 0.8520 |
| Regurgitation | 29.8±6.1 | 24.5±6.4 | 0.3365 |
| Vomiting | 14.0±4.7 | 8.8±4.9 | 0.5702 |
| Belching | 47.0±6.0 | 25.2±6.3 | 0.0121 |
| Dysphagia | 7.5±5.0 | 14.7±5.2 | 0.7421 |
| Epigastric pain | 15.8±5.5 | 16.7±5.8 | 0.9223 |
| LA grade of erosive esophagitis [n (%)] | 0.6617 | ||
| A | 15 (60) | 11 (47.8) | |
| B | 7 (28) | 7 (30.4) | |
| C | 2 (8) | 2 (8.7) | |
| D | 1 (4) | 3 (13.0) | |
| Hp infection status | 10 (40) | 11 (47.8) | 0.5643 |
VAS: visual analog scale, scored from 0 (none) to 100 (most severe); Hp: Helicobacter pylori; LA: Los Angeles.
Efficacy
The EE healing rates of esomeprazole and omeprazole treatment judged at the end of 8-wk trial [per-protocol (PP)] were 72.7% (16/22, 95%CI: 49.8-89.3%) and 50.0% (10/20, 27.2-72.8%) respectively [intent-to-treat (ITT): 64% (16/25, 95%CI: 44.3-83.8%) vs 45.5% (10/22, 95%CI: 22.7-68.3%), P = 0.2481], while the odds ratio was 2.667 (PP: 95%CI: 0.739-9.63, P = 0.2040) for esomeprazole over omeprazole.
In order to understand whether esomeprazole was effective in reducing LA-based EE grading, we further analyzed their extent of changed grading for both groups, e.g., 0 (no change), -1 (A to healed, B to A, C to B and D to C), -2 (B to healed, C to A and D to B) and -3 (C to healed, D to A), respectively (Figure 1). Although esomeprazole showed a better healing ability, however, no significant difference was found.
Figure 1.
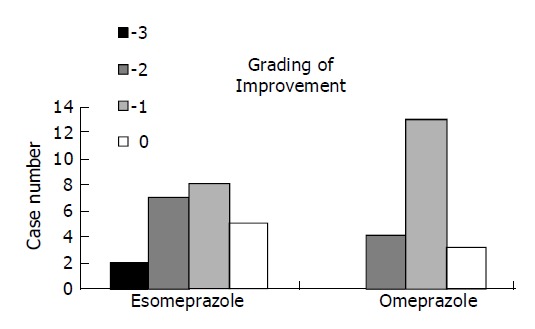
Histogram showing endoscopic assessment for the improved LA grading in erosive esophagitis patients after esomeprazole or omeprazole treatment. Grade of improvement: -3 means D to A or C to healed, -2 means D to B, C to A, or B to healed, -1 means D to C, C to B, B to A, or A to healed.
The median time to the first relief of heartburn between esomeprazole and omeprazole groups was similar on d 1 after treatment. On d 1, 77.3% and 65% of EE patients recorded the first relief of heartburn, respectively (Figure 2). Table 2 denotes that esomeprazole treatment was not significantly different from omeprazole in any of improved GERD symptom scores evaluated by patients themselves based on VAS except belching improvement was marked in patients undergoing esomeprazole treatment (P<0.05). Table 3 illustrates that the therapeutic symptomatic response scores of both treatments were similar (NS).
Figure 2.
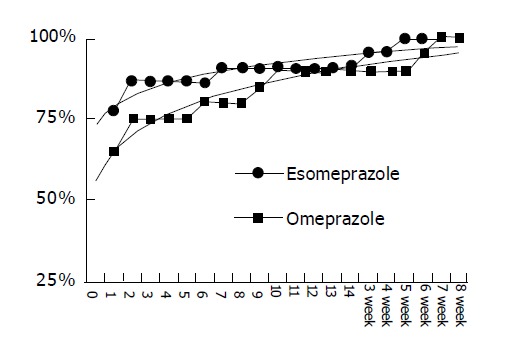
No cumulative percentage difference in relieving heartburn between erosive esophagitis patients after esomeprazole or omeprazole treatment illustrated by scattering plot.
Table 2.
Changes of reflux symptoms assessed on visual analog scale of studied patients 8 wk after esomeprazole or omeprazole treatment (mean±SE).
| Reflux symptoms | Esomeprazole n = 22 | Omeprazole n = 22 | P |
| Heartburn | -22.3±2.1 | -21.4±2.2 | 0.5453 |
| Nausea | -11.9±2.2 | -12.7±2.4 | 0.8867 |
| Regurgitation | -22.4±2.2 | -20.4±2.3 | 0.8598 |
| Vomiting | -9.1±1.61 | -8.8±1.7 | 0.4438 |
| Belching | -24.1±4.3 | -17.9±4.6 | 0.0113 |
| Dysphagia | -8.4±1.4 | -6.5±1.5 | 0.8044 |
| Epigastric pain | -10.6±2.0 | -11.1±2.1 | 0.1747 |
VAS was scored from 0 (none) to 100 (most severe).
Table 3.
Changed reflux symptoms of studied patients 8 wk after esomeprazole or omeprazole treatment (%).
| Esomeprazole (n = 22) | Omeprazole (n = 20) | P | ||
| Heartburn | Improved | 50.0 | 65.0 | 0.0993 |
| No change | 50.0 | 25.0 | ||
| Worse | 0.0 | 10 | ||
| Regurgitation | Improved | 77.3 | 85.0 | 1.0000 |
| No change | 18.2 | 15.0 | ||
| Worse | 4.5 | 0.0 | ||
| Dysphagia | Improved | 36.4 | 35.0 | 0.8697 |
| No change | 63.6 | 60.0 | ||
| Worse | 0.0 | 5 | ||
| Epigastric pain | Improved | 27.3 | 50.0 | 0.1895 |
| No change | 63.6 | 50.0 | ||
| Worse | 9.1 | 0.0 | ||
| Nausea | Improved | 22.7 | 35.0 | 0.5036 |
| No change | 68.2 | 65.0 | ||
| Worse | 9.1 | 0 | ||
| Vomiting | Improved | 22.7 | 40.0 | 0.3200 |
| No change | 77.3 | 60.0 | ||
| Worse | 0.0 | 0.0 | ||
| Belching | Improved | 54.5 | 45.0 | 0.8999 |
| No change | 36.4 | 45.0 | ||
| Worse | 9.1 | 10 |
Safety
In general, EE patients receiving esomeprazole (28.0%) and omeprazole (26.1%) treatment reported AE at least once during the trial (NS). Among them, constipation, dry skin sensation, diarrhea, headache, somnolence, etc., were the recorded AE in both groups (Table 4). Their distributions were also not different. Only one patient in omeprazole group reported a serious adverse event of cellulites during the treatment period, this causal relationship to treatment was judged to be unrelated. In addition, there were no clinically meaningful differences between treatment groups in terms of changed laboratory values or physical examinations.
Table 4.
AE in studied patients 8 wk after esomeprazole or omeprazole treatment (ITT).
| Esomeprazole (n = 25) n(%) | Omeprazole (n = 23) n (%) | P | |
| Patient with at least one AE | 7 (28.0) | 6 (26.1) | 1.0000 |
| Constipation | 2 (8.0) | 1 (4.4) | 1.0000 |
| Dry skin | 1 (4.0) | 3 (13.0) | 0.3381 |
| Diarrhea | 1 (4.0) | 1 (4.4) | 1.0000 |
| Headache | 1 (4.0) | 1 (4.4) | 1.0000 |
| Somnolence | 1 (4.0) | 1 (4.4) | 1.0000 |
| Cellulites | 0 (0.0) | 1 (4.4) | 0.4792 |
| Bronchitis | 0 (0.0) | 1 (4.4) | 0.4792 |
DISCUSSION
Our study mainly indicated that both esomeprazole and omeprazole were similarly effective in healing EE, relieving reflux symptoms for the Chinese EE patients in Taiwan. Gastro-esophageal reflux-induced EE is one of the GERDs, which ranges from endoscopy negative reflux to severe complications of Barrett’s esophagus as either high-grade dysplasia or adenocarcinoma[19,20]. Unlike endoscopy negative reflux, EE is very easily identified in GERD subjects based on experienced endoscopy. Until now, EE treatment is similar to any kind of GERD, e.g., reducing acid reflux, healing erosive lesions and preventing future relapse[2].
The EE severity is usually related to the extent and time of esophageal acid exposure[22]. It means the greater the acid exposure the severe the mucosal damage. Among the refluxed contents, acid is the most important pathogen leading to GERD, while effective acid reduction remains the only available method to treat GERD at this moment[2,22,23]. Accordingly, effective acid control for GERD subjects likely results in faster resolution of reflux symptoms, healing of reflux lesions quickly, better response of those with severe lesions and less frequent relapse. Symptom relief is indeed very important to all GERD patients because these symptoms usually bother their daily quality of life. Now step down treatment for GERD patients beginning with an effective PPI was recommended by the Genval consensus[2]. Although many of our studied GERD patients had mild EE, however, they still complained of cardinal reflux symptoms and other reflux-related symptoms. After 1 d of active esomeprazole and omeprazole treatment, 77.3% and 65% of patients recorded their first relief of heartburn. After 8-wk treatment, the VAS scores of many reflux symptoms were improved in both groups. In addition, the objective ranking of overall therapeutic effect showed a favorable and comparable result in both groups. We thus confirmed that 40 mg esomeprazole and 20 mg omeprazole daily for 8 wk could offer a sufficient acid suppression leading to the effective symptomatic relief for Asian EE patients without serious AE.
Benefits have been demonstrated in studies of esomeprazole vs omeprazole and lansoprazole[15,25-27]. Our study was to compare the therapeutic efficacy of a single isomer PPI, esomeprazole and omeprazole in treating EE patients in Taiwan. In fact, we found that EE was finally healed in more than half of our enrolled patients after 8-wk treatment. This EE healing rate was obviously lower than that in previous reports (67-85%) using PPI for a similar duration[5-7,28-33]. It has been pointed out that EE is usually less commonly presented and milder in nature among the Orientals in comparison with Occidentals[28-35]. In our study, the EE severity scored via LA grading system among the 48 consecutively enrolled patients was mainly classified as LA grade A, whereas previous reports from Western studies often included EE patients with an advanced grade[5-7,15-18,22-24]. Theoretically, our study should provide a better efficacy since many of the enrolled subjects had mild EE in nature. Surprisingly, we obtained a lower EE healing efficacy based on the similar PPI for a similar therapeutic duration. It is unknown whether the ethnic factor compromises the therapeutic efficacy. For example, PPI treatment for GERD patients only achieved 57.7% and 77% healing rates in two Japanese group studies, respectively[36,37]. Because our study was a single center trial and only enrolled a limited number of eligible EE patients, we believe that the lower and indistinguishable efficacy of esomeprazole vs omeprazole treatment was most likely originated from a type II error of inadequate study power, which was common in many drug trial studies[38,39]. In addition, long-term management of EE patients with PPI maybe more meaningful since initial management of EE patients to achieve healing has been overemphasized[40].
In summary, esomeprazole is at least similar to omeprazole in healing EE and removing reflux-related symptoms. Both esomeprazole and omeprazole are safe and well tolerated by Asian EE patients.
ACKNOWLEDGMENTS
The authors thank Jack Chai, MS, for his secretarial assistance.
Footnotes
Supported by the Research Foundation of Digestive Medicine, Taiwan, China
Science Editor Wang XL Language Editor Elsevier HK
References
- 1.Dodds WJ, Hogan WJ, Helm JF, Dent J. Pathogenesis of reflux esophagitis. Gastroenterology. 1981;81:376–394. [PubMed] [Google Scholar]
- 2.An evidence-based appraisal of reflux disease management-- the Genval Workshop Report. Gut. 1999;44 Suppl 2:S1–S16. doi: 10.1136/gut.44.2008.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tytgat GN, Nio CY. The medical therapy of reflux oesophagitis. Baillieres Clin Gastroenterol. 1987;1:791–807. doi: 10.1016/0950-3528(87)90019-4. [DOI] [PubMed] [Google Scholar]
- 4.Bell NJ, Hunt RH. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut. 1992;33:118–124. doi: 10.1136/gut.33.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bate CM, Green JR, Axon AT, Murray FE, Tildesley G, Emmas CE, Taylor MD. Omeprazole is more effective than cimetidine for the relief of all grades of gastro-oesophageal reflux disease-associated heartburn, irrespective of the presence or absence of endoscopic oesophagitis. Aliment Pharmacol Ther. 1997;11:755–763. doi: 10.1046/j.1365-2036.1997.00198.x. [DOI] [PubMed] [Google Scholar]
- 6.Galmiche JP, Barthelemy P, Hamelin B. Treating the symptoms of gastro-oesophageal reflux disease: a double-blind comparison of omeprazole and cisapride. Aliment Pharmacol Ther. 1997;11:765–773. doi: 10.1046/j.1365-2036.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 7.Hallerbäck B, Unge P, Carling L, Edwin B, Glise H, Havu N, Lyrenäs E, Lundberg K. Omeprazole or ranitidine in long-term treatment of reflux esophagitis. The Scandinavian Clinics for United Research Group. Gastroenterology. 1994;107:1305–1311. doi: 10.1016/0016-5085(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 8.Lu CL, Chen TS, Chen CY, Chang FY, Kang LJ, Lee SD. Treatment of erosive oesophagitis with omeprazole: a comparison with different delivery system. Dig Liver Dis. 2001;33:731. doi: 10.1016/s1590-8658(01)80052-9. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg P, Keeling D, Fryklund J, Andersson T, Lundborg P, Carlsson E. Review article: Esomeprazole--enhanced bio-availability, specificity for the proton pump and inhibition of acid secretion. Aliment Pharmacol Ther. 2003;17:481–488. doi: 10.1046/j.1365-2036.2003.01481.x. [DOI] [PubMed] [Google Scholar]
- 10.Sjövall H, Björnsson E, Holmberg J, Hasselgren G, Röhss K, Hassan-Alin M. Pharmacokinetic study of esomeprazole in patients with hepatic impairment. Eur J Gastroenterol Hepatol. 2002;14:491–496. doi: 10.1097/00042737-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 11.1999 [Google Scholar]
- 12.Lind T, Rydberg L, Kylebäck A, Jonsson A, Andersson T, Hasselgren G, Holmberg J, Röhss K. Esomeprazole provides improved acid control vs. omeprazole In patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14:861–867. doi: 10.1046/j.1365-2036.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- 13.Andersson T, Hassan-Alin M, Hasselgren G, Röhss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet. 2001;40:411–426. doi: 10.2165/00003088-200140060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kahrilas PJ, Falk GW, Johnson DA, Schmitt C, Collins DW, Whipple J, D'Amico D, Hamelin B, Joelsson B. Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther. 2000;14:1249–1258. doi: 10.1046/j.1365-2036.2000.00856.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang CS, Poon SK, Lien HC, Chen GH. The incidence of reflux esophagitis among the Chinese. Am J Gastroenterol. 1997;92:668–671. [PubMed] [Google Scholar]
- 16.Goh KL, Chang CS, Fock KM, Ke M, Park HJ, Lam SK. Gastro-oesophageal reflux disease in Asia. J Gastroenterol Hepatol. 2000;15:230–238. doi: 10.1046/j.1440-1746.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 17.Kao AW, Sheu BS, Sheu MJ, Chang YM, Huang SF, Chuang CH, Lai YL, Kao YH. On-demand therapy for Los Angeles grade A and B reflux esophagitis: esomeprazole versus omeprazole. J Formos Med Assoc. 2003;102:607–612. [PubMed] [Google Scholar]
- 18.Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 19.Jankowski JA, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the west. Gastroenterology. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 20.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett's metaplasia. Lancet. 2000;356:2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 21.Whittles CE, Biddlestone LR, Burton A, Barr H, Jankowski JA, Warner PJ, Shepherd NA. Apoptotic and proliferative activity in the neoplastic progression of Barrett's oesophagus: a comparative study. J Pathol. 1999;187:535–540. doi: 10.1002/(SICI)1096-9896(199904)187:5<535::AID-PATH302>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Richter JE. Gastroesophageal reflux disease in the older patient: presentation, treatment, and complications. Am J Gastroenterol. 2000;95:368–373. doi: 10.1111/j.1572-0241.2000.t01-1-01791.x. [DOI] [PubMed] [Google Scholar]
- 23.Dent J. Gastro-oesophageal reflux disease. Digestion. 1998;59:433–445. doi: 10.1159/000007521. [DOI] [PubMed] [Google Scholar]
- 24.Pope CE. Acid-reflux disorders. N Engl J Med. 1994;331:656–660. doi: 10.1056/NEJM199409083311007. [DOI] [PubMed] [Google Scholar]
- 25.Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, Skammer W, Levine JG. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol. 2002;97:575–583. doi: 10.1111/j.1572-0241.2002.05532.x. [DOI] [PubMed] [Google Scholar]
- 26.Lauritsen K, Devière J, Bigard MA, Bayerdörffer E, Mózsik G, Murray F, Kristjánsdóttir S, Savarino V, Vetvik K, De Freitas D, et al. Esomeprazole 20 mg and lansoprazole 15 mg in maintaining healed reflux oesophagitis: Metropole study results. Aliment Pharmacol Ther. 2003;17:333–341. doi: 10.1046/j.1365-2036.2003.01464.x. [DOI] [PubMed] [Google Scholar]
- 27.Vakil NB, Shaker R, Johnson DA, Kovacs T, Baerg RD, Hwang C, D'Amico D, Hamelin B. The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6-month, randomized, double-blind, placebo-controlled study of efficacy and safety. Aliment Pharmacol Ther. 2001;15:927–935. doi: 10.1046/j.1365-2036.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- 28.Gough AL, Long RG, Cooper BT, Fosters CS, Garrett AD, Langworthy CH. Lansoprazole versus ranitidine in the maintenance treatment of reflux oesophagitis. Aliment Pharmacol Ther. 1996;10:529–539. doi: 10.1046/j.1365-2036.1996.14156000.x. [DOI] [PubMed] [Google Scholar]
- 29.Hatlebakk JG, Berstad A, Carling L, Svedberg LE, Unge P, Ekström P, Halvorsen L, Stallemo A, Hovdenak N, Trondstad R. Lansoprazole versus omeprazole in short-term treatment of reflux oesophagitis. Results of a Scandinavian multicentre trial. Scand J Gastroenterol. 1993;28:224–228. doi: 10.3109/00365529309096076. [DOI] [PubMed] [Google Scholar]
- 30.Castell DO, Richter JE, Robinson M, Sontag SJ, Haber MM. Efficacy and safety of lansoprazole in the treatment of erosive reflux esophagitis. The Lansoprazole Group. Am J Gastroenterol. 1996;91:1749–1757. [PubMed] [Google Scholar]
- 31.Mee AS, Rowley JL. Rapid symptom relief in reflux oesophagitis: a comparison of lansoprazole and omeprazole. Aliment Pharmacol Ther. 1996;10:757–763. doi: 10.1046/j.1365-2036.1996.56198000.x. [DOI] [PubMed] [Google Scholar]
- 32.Robinson M, Sahba B, Avner D, Jhala N, Greski-Rose PA, Jennings DE. A comparison of lansoprazole and ranitidine in the treatment of erosive oesophagitis. Multicentre Investigational Group. Aliment Pharmacol Ther. 1995;9:25–31. doi: 10.1111/j.1365-2036.1995.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 33.Mössner J, Hölscher AH, Herz R, Schneider A. A double-blind study of pantoprazole and omeprazole in the treatment of reflux oesophagitis: a multicentre trial. Aliment Pharmacol Ther. 1995;9:321–326. doi: 10.1111/j.1365-2036.1995.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 34.Xia HH, Talley NJ. Helicobacter pylori infection, reflux esophagitis, and atrophic gastritis: an unexplored triangle. Am J Gastroenterol. 1998;93:394–400. doi: 10.1111/j.1572-0241.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- 35.Bercík P, Verdú EF, Armstrong D. H pylori related increase in omeprazole (ome) effect is associated with ammonia production (abstr) Gastroenterology. 1996;110:A64. [Google Scholar]
- 36.Endo M, Sugihara K. Long-term maintenance treatment of reflux esophagitis resistant to H2-RA with PPI (lansoprazole) Nihon Rinsho. 2000;58:1865–1870. [PubMed] [Google Scholar]
- 37.Umeda N, Miki K, Hoshino E. Lansoprazole versus famotidine in symptomatic reflux esophagitis: a randomized, multicenter study. J Clin Gastroenterol. 1995;20 Suppl 1:S17–S23. doi: 10.1097/00004836-199506001-00005. [DOI] [PubMed] [Google Scholar]
- 38.Talley N. Managing reflux disease-Critically reviewing the evidence in 2003. International Symposium, Marbella, Spain, January 18. 2003 [Google Scholar]
- 39.Al MJ, van Hout BA, Michel BC, Rutten FF. Sample size calculation in economic evaluations. Health Econ. 1998;7:327–335. doi: 10.1002/(sici)1099-1050(199806)7:4<327::aid-hec342>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 40.Dent J, Talley NJ. Overview: initial and long-term management of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17 Suppl 1:53–57. doi: 10.1046/j.1365-2036.17.s1.10.x. [DOI] [PubMed] [Google Scholar]


