Abstract
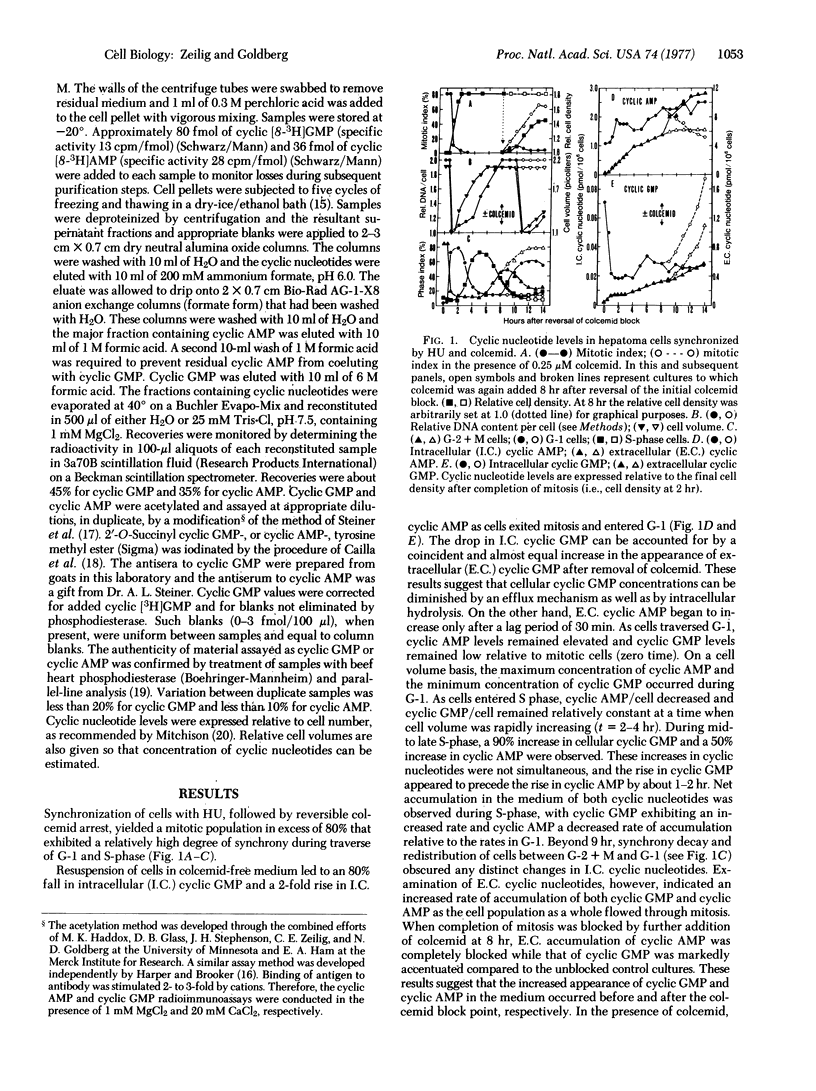
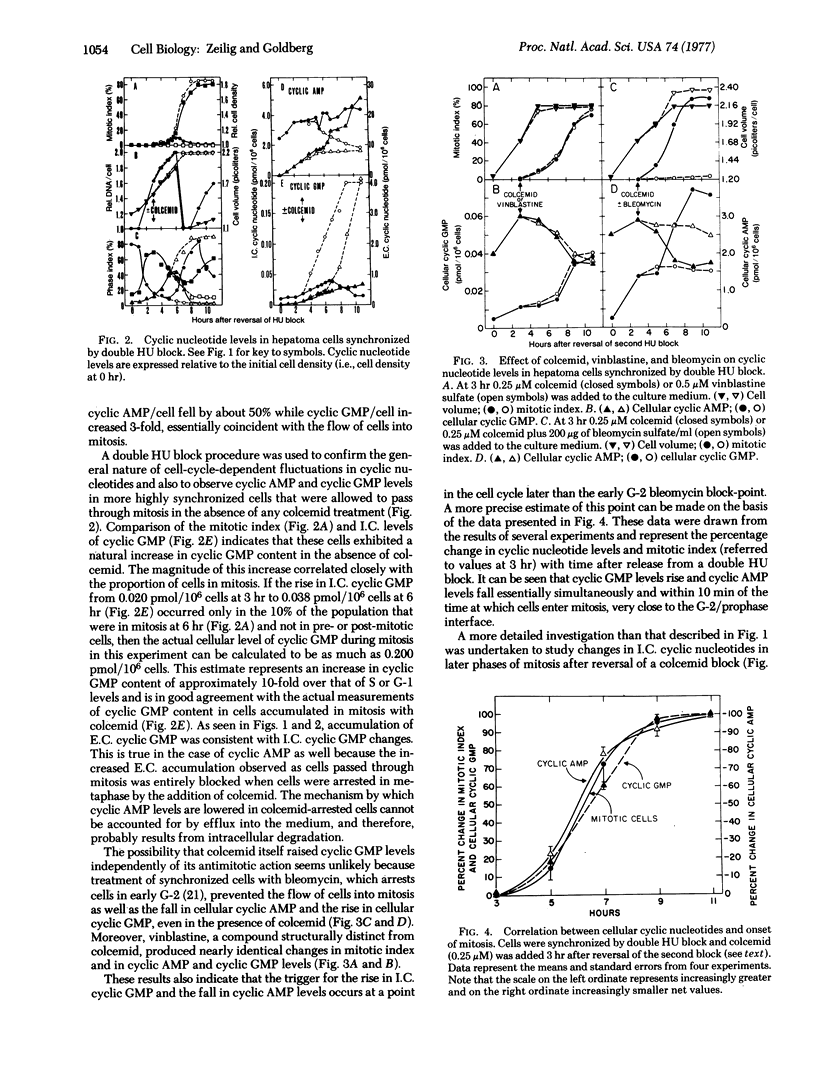
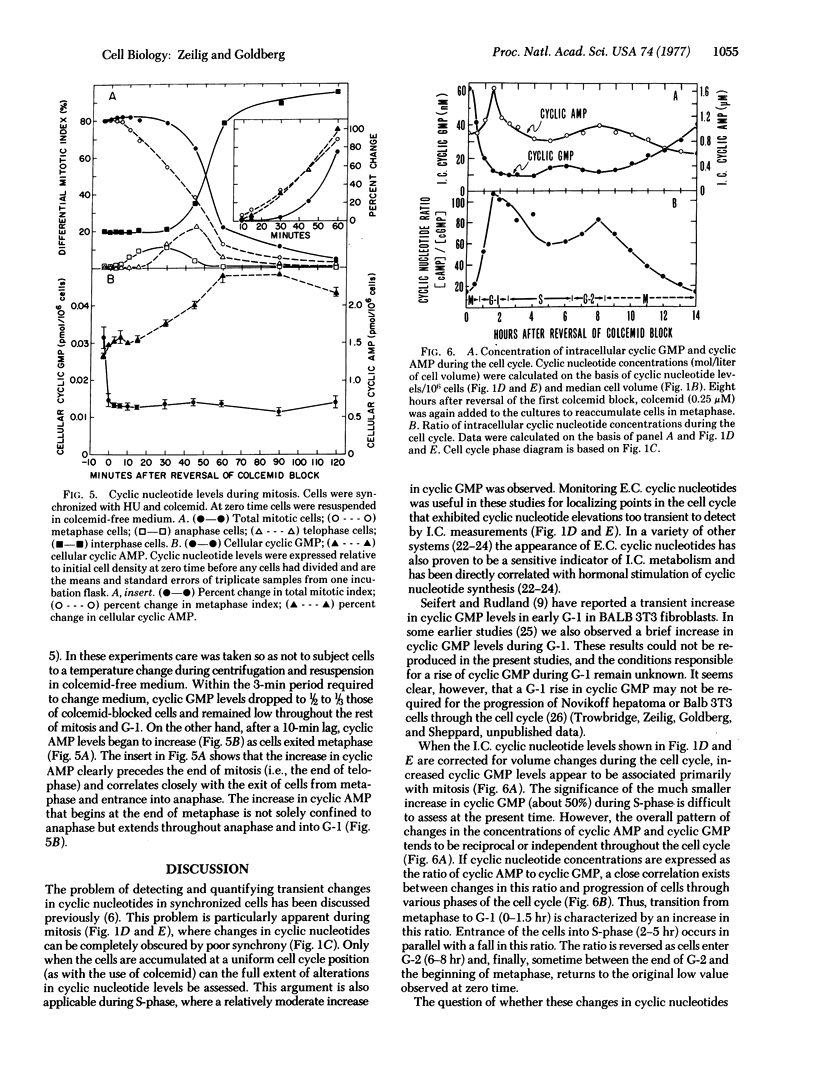
Intracellular and extracellular levels of 3':5'-cyclic GMP and 3':5'-cyclic AMP were studied in synchronized Novikoff rat hepatoma cells. Intracellular levels of cyclic GMP increased spontaneously from 2-fold (without colcemid) to 10-fold (with colcemid), in proportion to the number of cells in mitosis. As cells entered mitosis, cellular cyclic AMP declined simultaneously with the rise in cyclic GMP. These reciprocal changes in cyclic nucleotide levels were reversed as cells passed out of metaphase and through anaphase. Maximum cyclic AMP and minimum cyclic GMP concentrations occurred during G-1. Less marked reciprocal fluctuations in both cyclic nucleotides were also found in S-phase and early G-2, where the ratio of cyclic AMP to cyclic GMP concentrations first fell and then increased. These changes in cyclic nucleotide ratios were closely correlated with major cell-cycle transitions at the boundaries between G-1/S-phase, S-phase/G-2, G-2/prophase, and metaphase/anaphase. Most, but not all, of the extracellular cyclic nucleotides were extruded when cells traversed mitosis. Colcemid or vinblastine completely prevented the appearance of extracellular cyclic AMP but augmented the appearance of extracellular cyclic GMP in parallel with the accumulation of mitotic cells. These results reflected changes in intracellular cyclic nucleotides and indicated that increased intracellular turnover of cyclic GMP and cyclic AMP occurred before and after metaphase, respectively. Elevated cyclic GMP levels during mitosis and S-phase are consistent with potential modulatory roles for this cyclic nucleotide in proliferation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abell C. W., Monahan T. M. The role of adenosine 3',5'-cyclic monophosphate in the regulation of mammalian cell division. J Cell Biol. 1973 Dec;59(3):549–558. doi: 10.1083/jcb.59.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J. H., Kaminsky N. I., Hardman J. G., Broadus A. E., Sutherland E. W., Liddle G. W. Effects of catecholamines and adrenergic-blocking agents on plasma and urinary cyclic nucleotides in man. J Clin Invest. 1972 Aug;51(8):2124–2129. doi: 10.1172/JCI107019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailla H. L., Vannier C. J., Delaage M. A. Guanosine 3', 5'-cyclicmonophosphate assay at 10(-15)-mole level. Anal Biochem. 1976 Jan;70(1):195–202. doi: 10.1016/s0378-5173(83)90100-x. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVOREN P. R., SUTHERLAND E. W. THE EFFECT OF L-EPINEPHRINE AND OTHER AGENTS ON THE SYNTHESIS AND RELEASE OF ADENOSINE 3',5'-PHOSPHATE BY WHOLE PIGEON ERYTHROCYTES. J Biol Chem. 1963 Sep;238:3009–3015. [PubMed] [Google Scholar]
- Friedman D. L., Johnson R. A., Zeilig C. E. The role of cyclic nucleotides in the cell cycle. Adv Cyclic Nucleotide Res. 1976;7:69–114. [PubMed] [Google Scholar]
- Granner D., Chase L. R., Aurbach G. D., Tomkins G. M. Tyrosine aminotransferase: enzyme induction independent of adenosine 3', 5'-monophosphate. Science. 1968 Nov 29;162(3857):1018–1020. doi: 10.1126/science.162.3857.1018. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hirsch J., Haist B., Lutz D., Stacher A. Comparison of impulscytophotometric and autoradiographic methods with leukemic cells. Osterr Z Onkol. 1974;(2):47–50. [PubMed] [Google Scholar]
- Hochman J., Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. A structural gene mutation affecting the regulatory subunit of cyclic AMP-dependent protein kinase in mouse lymphoma cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5051–5055. doi: 10.1073/pnas.72.12.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Z., Lovelace E., Gallo M., Pastan I. Cyclic guanosine monophosphate and cellular growth. Science. 1975 Dec 19;190(4220):1213–1215. doi: 10.1126/science.173021. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Lawford H. G., Wong J. T. An improved procedure for extraction and analysis of cellular nucleotides. Anal Biochem. 1970 Jun;35(2):305–313. doi: 10.1016/0003-2697(70)90189-2. [DOI] [PubMed] [Google Scholar]
- Nesbitt J. A., 3rd, Anderson W. B., Miller Z., Pastan I., Russell T. R., Gospodarowicz D. Guanylate cyclase and cyclic guanosine 3':5'-monophosphate phosphodiesterase activities and cyclic guanosine 3':5'-monophosphate levels in normal and transformed fibroblasts in culture. J Biol Chem. 1976 Apr 25;251(8):2344–2352. [PubMed] [Google Scholar]
- Park C. R., Lewis S. B., Exton J. H. Relationship of some hepatic actions of insulin to the intracellular level of cyclic adenylate. Diabetes. 1972;21(2 Suppl):439–446. doi: 10.2337/diab.21.2.s439. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Plagemenn P. G., Richey D. P., Zylka J. M., Erbe J. Cell cycle and growth stage-dependent changes in the transport of nucleosides, hypoxanthine, choline, and deoxyglucose in cultured Novikoff rat hepatoma cells. J Cell Biol. 1975 Jan;64(1):29–41. doi: 10.1083/jcb.64.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Plagemann P. G. Growth of Novikoff rat hepatoma cells in suspension culture in the presence of adenosine 3',5'-cyclic monophosphate. J Natl Cancer Inst. 1971 Feb;46(2):423–429. [PubMed] [Google Scholar]
- Seifert W., Rudland P. S. Cyclic nucleotides and growth control in cultured mouse cells: correlation of changes in intracellular 3':5' cGMP concentration with a specific phase of the cell cycle. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4920–4924. doi: 10.1073/pnas.71.12.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard J. R., Prescott D. M. Cyclic AMP levels in synchronized mammalian cells. Exp Cell Res. 1972 Nov;75(1):293–296. doi: 10.1016/0014-4827(72)90554-x. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Tobey R. A. Arrest of Chinese hamster cells in G 2 following treatment with the anti-tumor drug bleomycin. J Cell Physiol. 1972 Apr;79(2):259–266. doi: 10.1002/jcp.1040790210. [DOI] [PubMed] [Google Scholar]
- Zeilig C. E., Johnson R. A., Sutherland E. W., Friedman D. L. Adenosine 3':5'-monophosphate content and actions in the division cycle of synchronized HeLa cells. J Cell Biol. 1976 Nov;71(2):515–534. doi: 10.1083/jcb.71.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]



