Abstract
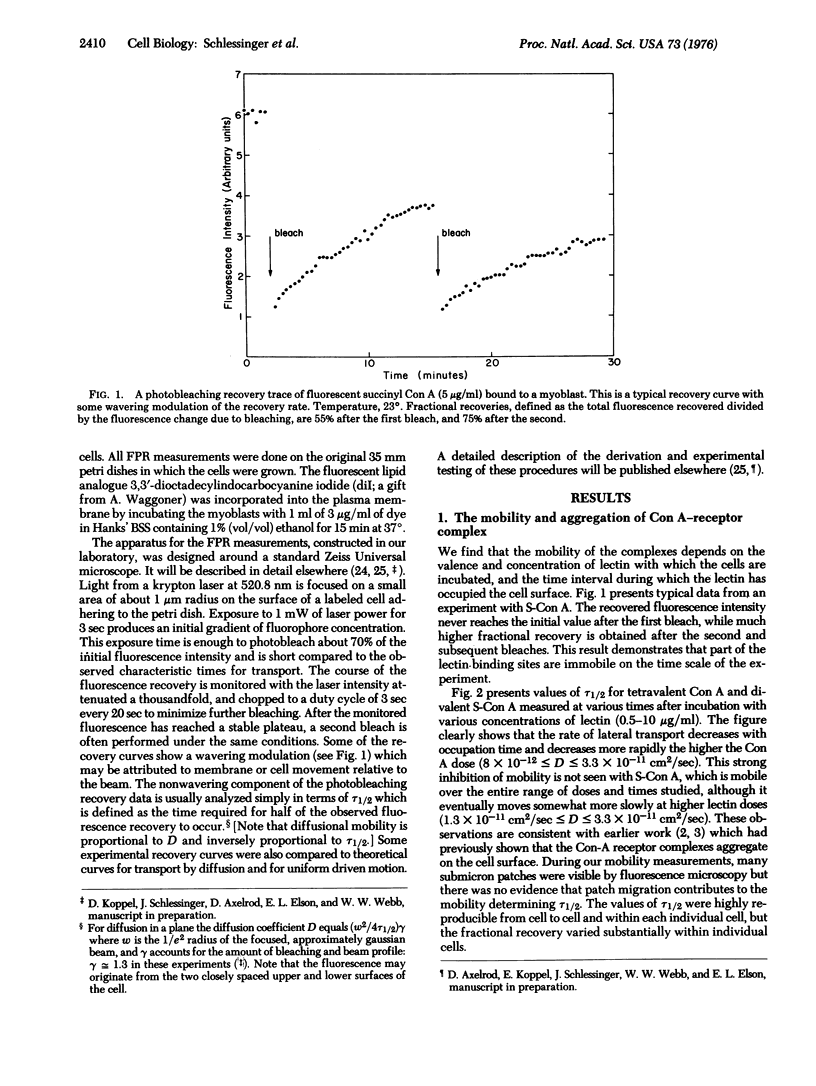
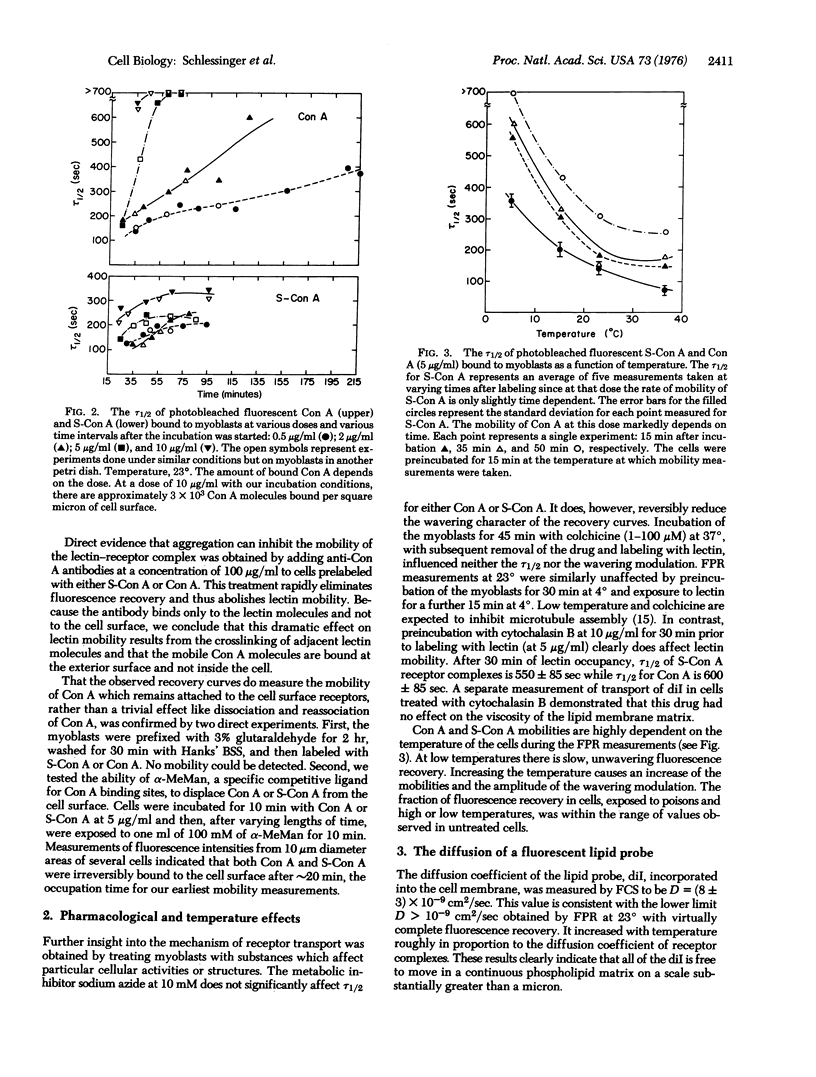
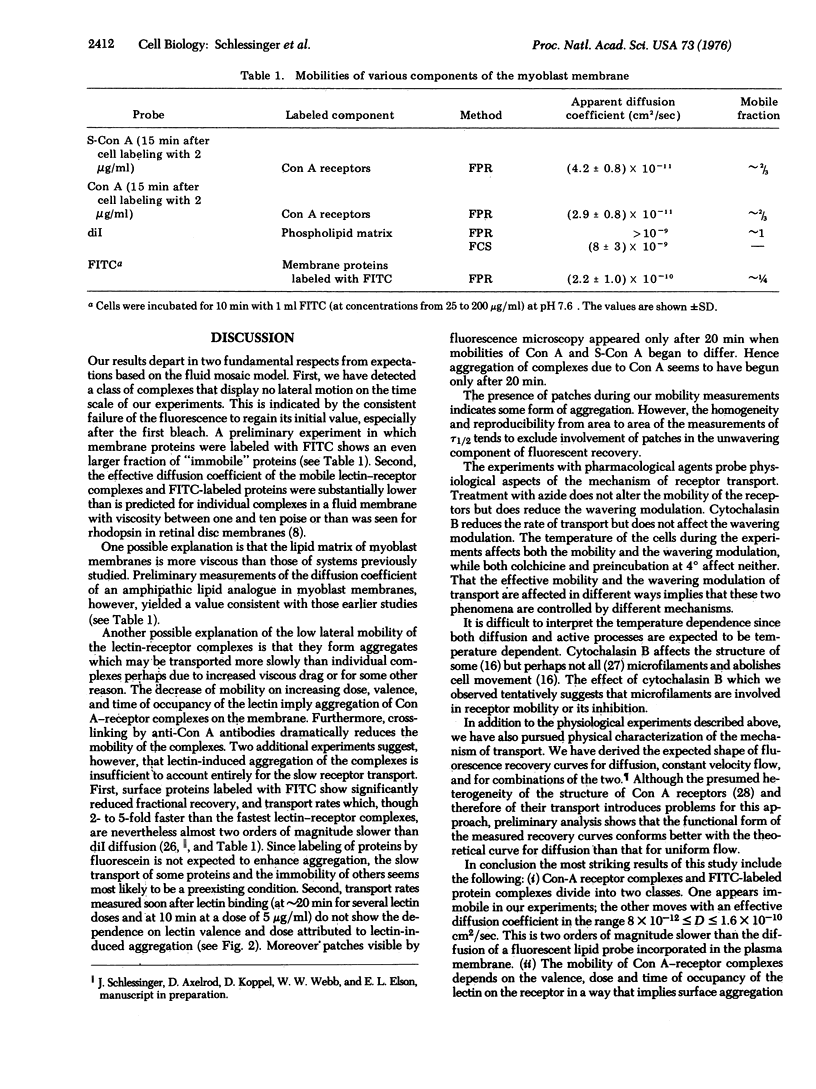
We report measurements of the lateral mobility of fluorescent labeled concanavalin A receptor complexes on the plasma membrane of cultured myoblasts of rat. Transport rates were measured by observing the recovery of fluorescence in a small region of the cell surface initially photobleached irreversibly by an intense, focused laser light pulse. Under different conditions we measured effective diffusion coefficients of the receptor complexes in the range 8 x 10(-12) less than D less than 3 x 10(-11) cm2/sec which is two orders of magnitude lower than we found for a fluorescent lipid probe, D approximately (8 +/- 3) x 10(-9) cm2/sec. This large difference and the presence of apparently immobile concanavalin A receptors suggests that factors beyond the fluoidity of the phospholipid bilayer membrane matrix control the rate of lateral transport of the complexes. Effective mobilities of the complexes decrease with increases in the valence, dose, and occupation time of the lectin on the membrane. These properties imply an aggregation of the lectin-receptor complexes. Mobilities are not influenced by azide, colchicine or preincubation at low temperature. Cytochalasin B and low temperatures, during the time of measurement, decrease the lateral transport rate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Protein mobility in membranes. FEBS Lett. 1975 Jul 15;55(1):1–7. doi: 10.1016/0014-5793(75)80943-4. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Kamin R. M., Fudenberg H. H. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J Immunol. 1969 Dec;103(6):1185–1195. [PubMed] [Google Scholar]
- Edidin M., Fambrough D. Fluidity of the surface of cultured muscle fibers. Rapid lateral diffusion of marked surface antigens. J Cell Biol. 1973 Apr;57(1):27–37. doi: 10.1083/jcb.57.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Webb W. W. Concentration correlation spectroscopy: a new biophysical probe based on occupation number fluctuations. Annu Rev Biophys Bioeng. 1975;4(00):311–334. doi: 10.1146/annurev.bb.04.060175.001523. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Petit V. A., Edidin M. Lateral phase separation of lipids in plasma membranes: effect of temperature on the mobility of membrane antigens. Science. 1974 Jun 14;184(4142):1183–1185. doi: 10.1126/science.184.4142.1183. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Sela B. A., Wang J. L., Edelman G. M. Isolation of lectins of different specificities on a single affinity adsorbent. J Biol Chem. 1975 Sep 25;250(18):7535–7538. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Distribution of immunoglobulin on the surface of mouse lymphoid cells as determined by immunoferritin electron microscopy. Antibody-induced, temperature-dependent redistribution and its implications for membrane structure. Eur J Immunol. 1972 Dec;2(6):523–535. doi: 10.1002/eji.1830020611. [DOI] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Normal distribution, patching and capping of lymphocyte surface immunoglobulin studied by electron microscopy. Nat New Biol. 1973 Feb 28;241(113):257–259. doi: 10.1038/newbio241257a0. [DOI] [PubMed] [Google Scholar]



