Abstract
AIM: To explore the effects of peptide S-8300 from shark liver (S-8300) on liver function as well as in regulating immune functions in experimental injury models.
METHODS: Mice were administered with different doses of S-8300 for four consecutive days, followed by mice injected with tetrachloromethane (CCl4) on d 3. The activity of ALT, AST, LDH, SOD and contents of MDA and GSH in the mice liver were tested. And mice were treated with Cy (100 mg/kg) at the beginning of the experiment to induce immunosuppression model. The S-8300 groups were treated with S-8300 seven days after the beginning of Cy administration. The effects of S-8300 on the formulation of serum hemolysin and the content of IL-2 in serum in the immunosuppression mice were observed.
RESULTS: S-8300 obviously decreased the level of ALT (52.2±11.0 U/dL vs 135.9±6.5 U/dL, P<0.01), AST (67.5±6.9 U/dL vs 238.8±8.7 U/dL, P<0.01), LDH (155.1±46.8 U/dL vs 240.4±6.0 U/dL, P<0.01) & MDA (0.64±0.027 nmol/mg vs 1.06±0.040 nmol/mg, P<0.01) and increased SOD (24.51±1.01 U/mg vs 19.05±0.73 U/mg, P<0.01) & GSH (24.17±0.91 µg/mg vs 14.93±0.45 µg/mg, P<0.01) in mice liver damaged by CCl4. S-8300 also markedly improved the formulation of serum hemolysin (0.094±0.005 A540 vs 0.063±0.006 A540, P<0.01) and increased the level of IL-2 (9.74±1.16 pg/mL vs 5.81±0.87 pg/mL, P<0.01) in serum of the immunosuppression mice.
CONCLUSION: The results suggested S-8300 has significant hepatoprotective, immunomodulatory and inhibiting of lipid peroxidation activity.
Keywords: CCl4, Immunosuppression, IL-2, Hepatoprotective, Serum hemolysin
INTRODUCTION
A number of investigators have isolated stimulatory factors from the liver of many animals, such as rats, mice and dogs[1]. Sharks were one of the most active animals in the ocean and their liver weighed over 70% of their total bowels with strong immunocompetence. We had reported that active peptide from shark liver had significant protective effects on CCl4 and D-galactosamine induced liver injury in mice[2] and immunoloregulation effect on immune liver injury in mice[3]. For exploring the functions of other active substances in shark livers, we isolated a new peptide S-8300 from shark liver. In the present study, we have reported that administration of S-8300 in CCl4 intoxicated hepatatoxic mice alters the serum marker enzyme and lipid peroxidation index levels, viz ALT, AST, LDH, SOD, GSH, MDA, and also modulates the levels of IL-2 and hemolysin in immunosuppression mice caused by cyclophosphamide. The hepatoprotective, immunomodulatory and inhibiting of lipid peroxidation activity of S-8300 was also reported. Therefore, the results of this study will be very helpful to recognize the beneficial effects of S-8300 on liver function as well as in regulating immune functions.
MATERIALS AND METHODS
Preparation of S-8300
S-8300 was extracted and purified from healthy squalus mitsukurii. The process of extraction and purification of S-8300 mainly included homogenization, heat treating, centrifugation, ultrafiltration, DEAE-sephadex chromatography, biogel P10 chromatography, FPLC mono Q chromatography and reversed-phase HPLC. It was estimated that the molecular weight of S-8300 was 8300 by SDS-PAGE[10].
Animals
The ICR mice weighing 20±2 g were obtained from the Experimental Animal Center of China Pharmaceutical University (Nanjing, China), and guinea pigs weighing 350-400 g were from Experimental Animal Center of Nanjing Medical University (Nanjing, China). All the animals were maintained with free access to pellet food and water in plastic cages at 22±2 °C, and kept on a 12 h light/dark cycle. This study complied with the current ethical regulations for animal research of this institute, and all mice used in the experiment received humane care.
Main reagents
Reagents employed in this study were as follows: Tetrachloromethane (CCl4, Nanjing 1st Chemical Reagents Company, Nanjing, China), Cyclophosphamide (Cy, Jiangsu Hengrui Pharmaceutical Co., Ltd., Nanjing, China), Hepatocellular growth factor (HGF, Nanda Pharmaceutical Co., Ltd., Nanjing, China), Sheep red blood cell (SRBC, Nanjing Agricultural University, Nanjing, China), IL-2 ELISA Kit (Jingmei Biotech Co., Ltd., Shenzhen, China), Alanine transaminase (ALT) measurement kit, Aspartate transaminase (AST) measurement kit, Lactate dehydrogenase (LDH) measurement kit, Superoxide dismutase (SOD) measurement kit, Maleic dialdehyde (MDA) measurement kit and Glutathione (GSH) measurement kit (Nanjing Jiangcheng Bioengineering Institute, Nanjing, China) were all used.
Liver injury model induced by CCl4
Mice were divided into six groups of 10 animals each. Group 1 was administrated with saline (20 mL/kg, i.p.) as a normal control. Group 2 was given CCl4/olive oil (1:100 v/v, 5 mL/kg, i.p.) as the treated control group. Group 3 received HGF (3 mg/kg, i.p.) as the standard reference group. Test groups of mice were treated with S-8300 at a dose of 0.3, 1 and 3 mg/kg body weight and injected for four consecutive days, followed by mice administrated CCl4/olive oil on d 3. At the end of the experimental period, all the animals were killed, blood was collected in sterile centrifuge tube and allowed to clot. Serum was separated by centrifuging at 2500 r/min for 15 min. After the animals were killed, their livers were removed. A part of liver specimens were homogenized in Tris-HCl buffer (0.01 mol/L, pH 7.4) using a homogenizer to give a 10% homogenate, the other liver sections were preserved in 10% neutral formalin, and were processed for paraffin embedding, following the standard microtechnique. Five micron sections of livers, stained with alum hematoxylin and eosin were observed under microscope for the histopathological changes.
Immunosuppression model caused by Cy
Mice were randomly allocated into a normal control group, a model control group, and three doses of S-8300 groups (0.3, 1 and 3 mg/kg) of 10 animals each. The latter four groups were administered with Cy (100 mg/kg) at the beginning of the experiment to induce immunosuppression model. The S-8300 groups were also treated with S-8300 seven days after the beginning of Cy administration. All control groups were given corresponding placebo at the same time. Mice were sensitized by injected 0.2 mL of 20% SRBC under the skin at d 2. At the end of the seven days, 2 h after the S-8300 injection, mice in each group were killed. They were bled and the blood was collected for serum hemolysin test and IL-2 ELISA assay.
Biochemical and enzymatic estimations
The levels of serum ALT, AST, LDH, SOD, IL-2, content of MDA and GSH in liver were determined by using commercial kits according to the guidelines indicated.
Determination of serum hemolysin
Serum hemolysin was determined using Ying et al[4]’s method and was measured spectrophotometrically at 540 nm.
Statistical analysis
Data were expressed as mean±SD. Statistical analysis was evaluated by one-way analysis of variance, followed by the Student-Newman-Kanul’s test for multiple comparisons which was used to evaluate the difference between two groups. P<0.01 was considered significant.
RESULTS
Alters of liver function
Administration of CCl4 to mice caused significant increase in serum marker enzymes like ALT (135.9±6.5 U/dL vs 13.4±2.5 U/dL, P<0.01), AST (238.8±8.7U/dL vs 42.4±10.9 U/dL, P<0.01), LDH (240.4±6.0 U/dL vs 150.3±45.8 U/dL, P<0.01), compared to normal control mice. Treatment with S-8300 caused significant reduction of these values (Table 1), ALT (52.2±11.0 U/dL vs 135.9±6.5 U/dL, P<0.01), AST (67.5±6.9 U/dL vs 238.8±8.7 U/dL, P<0.01), LDH (155.1±46.8 U/dL vs 240.4±6.0 U/dL, P<0.01), dose-dependently. The LDH level was almost restored to the levels found in control and S-8300 3 mg/kg group of mice. Treatment with HGF also reversed the hepatotoxicity significantly.
Table 1.
Effect of S-8300 on the sGPT, sGOT and LDH in mice injected with CCl4.
| Group | Dosage (mg/kg) | sGPT (U/dL) | sGOT (U/dL) | LDH (U/dL) |
| Normal control | – | 13.4±2.5b | 42.4±10.9b | 150.3±45.8b |
| Model control | – | 135.9±6.5 | 238.8±8.7 | 240.4±6.0 |
| HGF | 3 | 96.1±12.6b | 163.8±14.2b | 175±52.8b |
| S-8300 | 0.3 | 98.2±12.5b | 142.1±31.5b | 196.1±59.6b |
| 1 | 78.7±10.7b | 102.0±14.0b | 173.1±52.4b | |
| 3 | 52.2±11.0b | 67.5±6.9b | 155.1±46.8b |
P<0.01 vs model control.
Inhibition of lipid peroxidation
S-8300 was effective in inhibiting the lipid peroxidation induced by administration with CCl4. The lever of MDA (0.64±0.027 nmol/mg vs 1.06±0.040 nmol/mg, P<0.01) was significantly decreased and SOD (24.51±1.01 U/mg vs 19.05±0.73 U/mg, P<0.01) & GSH (24.17±0.91 µg/mg vs 14.93±0.45 µg/mg, P<0.01) increased in mice liver homogenate in a dose-dependent manner (Table 2). Treatment with HGF also reversed the lipid peroxidation significantly.
Table 2.
Effect of S-8300 on the GSH, SOD and MDA in mice injected with CCl4.
| Group | Dosage (mg/kg) | GSH (µg/mg prot) | SOD (U/mg prot) | MDA (nmol/mg prot) | General protein (mg/mL) |
| Normal control | – | 27.75±0.86b | 26.10±0.81b | 0.55±0.025b | 21.22±0.39 |
| Model control | – | 14.93±0.45 | 19.05±0.73 | 1.06±0.040 | 21.13±0.35 |
| HGF | 3 | 19.02±0.42b | 21.82±0.82b | 0.89±0.038b | 21.28±0.49 |
| S-8300 | 0.3 | 17.40±0.10b | 21.19±0.69b | 0.91±0.035b | 21.3±0.29 |
| 1 | 20.01±0.74b | 23.39±0.42b | 0.81±0.026b | 21.11±0.44 | |
| 3 | 24.17±0.91b | 24.51±1.01b | 0.64±0.027b | 21.3±0.43 |
P<0.01 vs model control.
Histological examination
Histological observations basically support the results obtained from serum enzyme assays. The liver of CCl4-intoxicated mice showed massive fatty changes, gross necrosis, broad infiltration of lymphocytes and Kupffer cells around the central vein and loss of cellular boundaries. The histological pattern of liver of mice pretreated with S-8300 and subsequently given CCl4 showed a more or less normal lobular pattern with a mild degree of fatty change, necrosis and lymphocyte infiltration almost comparable to the normal control and HGF groups (Figure 1).
Figure 1.
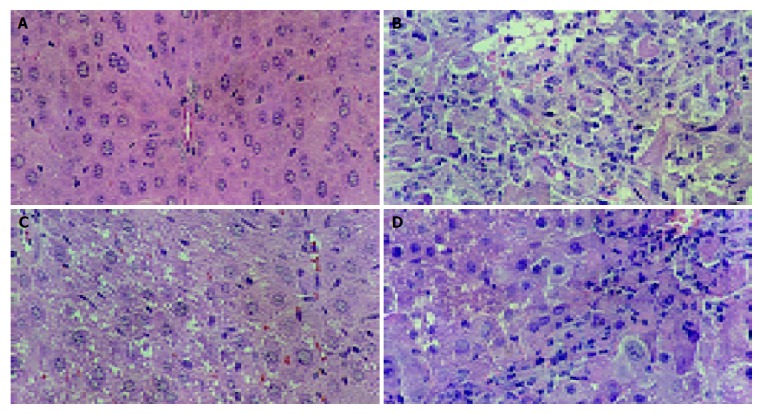
Hepatic histological findings of S-8300 against hepatic lesions induced by CCl4. A: Normal group, (H-E ×200); B: CCl4 alone, (H-E ×200); C: CCl4+S-8300 (3 mg/kg), (H-E×200); D: CCl4+HGF (3 mg/kg), (H-E ×200).
Immunomodulatory effect
S-8300 markedly improved the formulation of serum hemolysin (0.094±0.005 A540 vs 0.063±0.006 A540, P<0.01) and increased the lever of IL-2 (9.74±1.16 pg/mL vs 5.81±0.87 pg/mL, P<0.01) in serum of the immunosuppression mice (Table 3).
Table 3.
Effect of S-8300 on the levels of serum hemolysin and IL-2 in mice treated with Cy.
| Group | Dosage (mg/kg) | IL-2(pg/mL) | Serum hemolysin (A540) |
| Normal control | – | 12.72±1.62b | 0.101±0.007b |
| Model control | – | 5.81±0.87 | 0.063±0.006 |
| S-8300 | 0.3 | 7.44±078b | 0.082±0.003b |
| 1 | 8.19±0.95b | 0.084±0.004b | |
| 3 | 9.74±1.16b | 0.094±0.005b |
P<0.01 vs model control.
DISCUSSION
Liver tissue rich in both transaminase increased in patients with acute hepatic diseases, AST which is slightly elevated by cardiac necrosis is a more specific indicator of liver disease. In view of this, S-8300 mediated reduction in levels of AST, ALT and LDH towards the respective normal values is an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage caused by CCl4[7]. CCl4 is one of the most commonly used hepatotoxins in the experimental study of liver diseases. It has been established that CCl4 is accumulated in hepatic parenchyma cells and metabolically activated by cytochrome P450-dependent monooxygenases to form a trichloromethyl radical (CCl3)[8]. These activated radicals bind covalently to the macromolecules and induce peroxidative degradation of membrane lipids of endoplasmic reticulum rich in polyunsaturated fatty acids. This leads to the formation of lipid peroxides. This lipid peroxidative degradation of biomembranes is one of the principal causes of hepatotoxicity of CCl4[5]. Thus, antioxidant or free radical generation inhibition is important in protection against CCl4-induced liver lesions. The efficacy of any hepatoprotective drug is essentially dependent on its ability to reduce the harmful effects or maintain the normal hepatic physiology that has been disturbed by a hepatotoxin[6]. In the present study, administration of S-8300 not only decreased the CCl4 induced elevated levels of AST, ALT, LDH and MDA, but also increased levels of SOD and GSH. This suggested the maintenance of structural integrity of hepatocytic cell membrane or regeneration of damaged liver cells by inhibiting lipid peroxidation activity of S-8300. These findings can be further corroborated with histopathological studies. The histopathological examination clearly reveals that the hepatic cells, central vein, and portal triad are almost normal in S-8300 (3 mg/kg, i.p.) group in contrast to group which received CCl4. Thus, S-8300 can be considered to be an effective hepatoprotective peptide.
This work also evaluated the influence of S-8300 on activities in the immune system of mice experimentally induced immunosuppression. In the case of cellular immunity, the enhancement of IL-2 concentration in serum induced by S-8300 exerts profound changes in the number and activation degree of monocytes and T cells. In the case of humoral immune response, S-8300 could stimulate the formulation of hemolysin. The immunomodulatory effect of S-8300 on both parameters of cellular immune and humoral immune responses was effectively enhanced. S-8300 thus amplified an immune response.
The present study evaluated the association of liver protection by S-8300 and the modulation of the immune system suspected to be involved in the development of liver dysfunction. Hyperactivity of oxidants is thus equally as dangerous as hypoactivity, which may in turn cause immunosuppression[9]. It could be predicted that S-8300 could serve a beneficial role as an antioxidant, and was capable of removing free radicals from a system either by prolonging the initiation phase or by inhibiting the propagation phase of autooxidation. From our results, it could be suggested that S-8300 would be a good alternative to therapy for hepatitis. It would also scavenge the free radicals in human bodies, and with its immunostimulatory effect it could prevent foreign pathogenic invasion.
Footnotes
Supported by the National Hi-Tech Research and Development Program of China (863 Program), No. 2001AA624090 and 2003AA624090; the National Natural Science Foundation of China, No. 30171103; Youth Foundation of China Pharmaceutical University, No. C0316
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Francavilla A, Ove P, Polimeno L, Coetzee M, Makowka L, Rose J, Van Thiel DH, Starzl TE. Extraction and partial purification of a hepatic stimulatory substance in rats, mice, and dogs. Cancer Res. 1987;47:5600–5605. [PMC free article] [PubMed] [Google Scholar]
- 2.Guo Y, Sheng Q, Wu WT, Protective Effect of CPU-HAP-140 against Acute Liver Failure in Mice. Pharmaceutical Biotechnol. 2000;7:82–85. [Google Scholar]
- 3.Guo Y, Wu WT, Wu GZ. The liver protective and immuno modulative effect of Shaheptide in mice with immune liver injury. Chinese J New Drugs. 2001;10:29. [Google Scholar]
- 4.Ying HE, Jing cai LI, Yang Z, Feng XU. Modulation of melatonin on the immune fuctions in the light dark frequently shifting mice. J Shenyang Pharmaceutical University. 2004;21:126–129. [Google Scholar]
- 5.Rajkapoor B, Jayakar B, Kavimani S, Murugesh N. Effect of dried fruits of Carica papaya Linn on hepatotoxicity. Biol Pharm Bull. 2002;25:1645–1646. doi: 10.1248/bpb.25.1645. [DOI] [PubMed] [Google Scholar]
- 6.Raju K, Anbuganapathi G, Gokulakrishnan V, Rajkapoor B, Jayakar B, Manian S. Effect of dried fruits of Solanum nigrum LINN against CCl4-induced hepatic damage in rats. Biol Pharm Bull. 2003;26:1618–1619. doi: 10.1248/bpb.26.1618. [DOI] [PubMed] [Google Scholar]
- 7.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. J Ethnopharmacol. 2004;90:229–232. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Suja SR, Latha PG, Pushpangadan P, Rajasekharan S. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J Ethnopharmacol. 2004;92:61–66. doi: 10.1016/j.jep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Bishayi B, Roychowdhury S, Ghosh S, Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–146. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]
- 10.Feng-Jie H, Qian LI, Zheng-Bing LV, Wu-Tong WU. Protective Effect of Peptide S-8300 from Shark Liver on Acute Liver Injury in Mice. Zhongguo Haiyang Yaowu. 2004;97:17–20. [Google Scholar]


