Abstract
AIM: To investigate the effects of mitomycin (MMC) combined with sulindac on cell viability, apoptotic induction and expression of apoptosis-related gene Bcl-2 and cyclooxygenase-2 (COX-2) in gastric cancer SGC-7901 cells.
METHODS: Human gastric cancer SGC-7901 cells were divided into three treatment groups,namely sulindac treatment group, MMC treatment group and combined sulindac with MMC treatment group. After being treated with drugs, cell viability was examined by MTT assay. Flow cytometry was used to evaluate the cell cycle distribution and apoptotic rates. Morphology of the cells was observed under light microscope and interactive laser microscope. Expression of COX-2 and Bcl-2 was determined by immunocytochemical method.
RESULTS: After exposure for 12 h to three kinds of drugs, gastric cancer SGC-7901 cells presented some morphological features of apoptosis, including cell shrinkage, nuclear condensation, DNA fragmentation and formation of apoptotic bodies. Growth inhibition was more obvious in combined sulindac with MMC treatment group and sulindac treatment group than in MMC treatment group. The apoptotic rates in co-treated cells and MMC-treated cells 24 h after treatment were 12.0% and 7.2%, respectively. After exposure for 24 h to MMC, the expression of COX-2 and Bcl-2 protein was up-regulated, COX-2 levels were down-regulated but Bcl-2 gene expression was not changed significantly in combined treatment group.
CONCLUSION: MMC-induced apoptosis is reduced by up-regulating the expression of COX-2 and Bcl-2 genes. MMC combined with sulindac can suppress the growth of gastric cancer cells through induction of apoptosis mediated by down-regulation of apoptosis-related Bcl-2 and COX-2 gene.
Keywords: Apoptosis, Induced, Mitomycin with sulindac, Gastric cancer, SGC-7901
INTRODUCTION
The protein expressed by cyclooxygenase-2 (COX-2) gene is considered as a pro-oncogenic protein[1], and its overexpression in tumor tissue is related to the carcinogenesis and development of carcinoma[2-6]. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as aspirin, indomethacin and sulindac, may play a role in the inhibition of proliferation and induction of apoptosis of tumor cells through the inhibition of COX-2 activity[7-10]. Celecoxib, a selective COX-2 inhibitor of NSAIDs, approved by FDA has been used to reduce the number of adenomatous colorectal polyps in patients with familial adenomatous polyposis[11]. The present study was designed to observe the inhibitory effect of sulindac, mitomycin (MMC) and sulindac in combination with MMC on human gastric cancer SGC-7901 cells in vitro.
MATERIALS AND METHODS
Materials
Human gastric cancer cell line SGC-7901 was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. RPMI 1640 medium was a product of Gibco. Sulindac, MTT and propidium iodide (PI) were purchased from Sigma. MMC was purchased from Kyowa Hakko Kogko Co., Japan. Goat anti-human COX-2 multiclonal antibody was purchased from Santa Cruz Biotechnology. SP detection kit and Bcl-2 monoclonal antibody were from Maixing Biotech Co, Fujian, China.
Methods
Human gastric cancer SGC-7901 cells were divided into sulindac treatment groups, MMC treatment group, and combined sulindac with MMC treatment group. Then cells were treated with concentrations of 1 mmol/L sulindac, 1×10-3 g/L MMC and 1 mmol/L sulindac in combination with 1×10-3 g/L MMC, respectively. Cells were grown in RPMI 1640 supplemented with 100 mL/L fetal bovine serum (FBS), penicillin (100 mg/L) and streptomycin (100 mg/L) in a humidified atmosphere of 50 mL/L CO2 at 37 °C.
MTT assay
SGC-7901 cells were plated at 7×103 cells/well in 96-well plates in RPMI 1640 containing 10% FBS. After 24 h, the culture medium was replaced by a fresh medium containing the three drugs. Six duplicate wells were set up in each sample. The cells not treated with the drugs served as control cells. After 12-, 24- or 48-h incubation, 20 mL MTT (5 g/L) was added to each well and incubated for 4 h. Supernatant was then removed, 150 mL DMSO was added, then shaken for 10 min until the crystal was dissolved. A570 nm value was measured with an ELISA reader. The negative control well had no cells and was used as zero point of absorbance. Each assay was performed in triplicate. Cell growth curve was completed using time as the abscissa and A value (mean±SD) as the ordinate.
Morphological observation
After treated with drugs, cytologic morphological changes were observed under the Olympus optical microscope. Cells were subcultured on coverslips in 6-well culture plates. After 12, 24 and 48 h the coverslips were taken out and observed after stained with HE. A drop of cell suspension solution treated with drugs was added onto the cover glass slide and mixed with 5 µL acridine orange. After 10 min, cells were observed and photographs were taken under interactive laser microscope.
Flow cytometric analysis
SGC-7901 cells untreated and treated with drugs were collected, rinsed in PBS, resuspended and fixed in 70% ethanol at 4 °C overnight, then washed with PBS again, treated with 200 µL 100 mg/L RNase at 37 °C for 15 min and stained with 100 mL 50 mg/L PI at 4 °C for 30 min in darkness. Cell cycle distribution at different phases and apoptotic rate were analyzed by FACScan flow cytometry.
Immunocytochemical analysis of COX-2 and Bcl-2
Cells treated with different drugs were cultured on coverslips in a 6-well plate. After 24 h, the cells growing on coverslips were fixed with cold 950 mL/L ethanol for 30 min. After washing in PBS, the cells were incubated in 5 mL/L H2O2 solution to inactivate endogenous peroxidase, blocked with normal goat serum at room temperature for 10 min to reduce the non-specific binding, and incubated with monoclonal Bcl-2, multiclonal COX-2 antibodies at 4 °C overnight. Then cells on the coverslips were incubated with biotinylated anti-mouse IgG at 37 °C for 12 min, and then incubated in streptavidin-peroxidase at 37 °C for 15 min. The chromogenic reaction was developed with diaminobenzidine. PBS was used as substitute of protein antibody for negative controls.
RESULTS
Proliferation and apoptosis of SGC-7901 cells
Cell growth was determined by MTT assay. As shown in Figure 1, MMC combined with sulindac showed a more potent effect on the growth of reducing SGC-7901 cells than MMC alone. Under optical microscope, SGC-7901 cells untreated with drugs were rhomboidal or polygonal in shape, and stuck together to clusters. After treatment, some cells rounded up off the plate, exhibiting smaller and circular shape and suspending in culture medium. Through HE staining, SGC-7901 cells exhibited the characteristics of apoptosis including cell shrinkage, deep-dyed pyknotic nuclei, fragmentation of nuclei and apoptotic body formation. The viable cells, not dyeable to fluorescence agent, were stained shallowly (Table 1). Under interactive laser microscope, as shown in Figure 2, in the control group, the nuclei were big and round, and chromatin exhibiting a green fluorescence was disperse. However, cytoplasmic and nuclear shrinkage, increased density and margination of nuclear chromatin, cytoplasmic blebbing and apoptotic bodies were easily identified in drug-treated groups. As compared to other groups, combined treatment group had many more cells with apoptotic characteristics as described above. The longer the time treated with drugs, the more the apoptotic cells there were.
Figure 1.
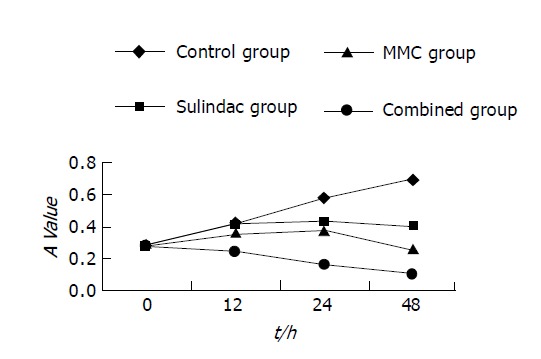
Inhibitory effect of three drugs on SGC-7901 cell.
Table 1.
Effect of drugs on apoptosis and cell cycle of SGC-7901 cell line (%, mean±SD).
| Group | Apoptosis | G0 /G1 | S | G2/M |
| Control | 0.43±0.38 | 47.43±2.81 | 45.50±1.25 | 7.06±4.06 |
| Sulindac | 3.80±0.53a | 49.67±3.29 | 44.70±5.82 | 4.97±6.52 |
| MMC | 7.20±1.31a | 56.67±3.92a | 40.40±11.53 | 6.60±2.99 |
| Combined | 11.9±2.59b | 68.40±5.53b | 29.23±5.87 | 2.37±0.45 |
P<0.05 vs control,
P<0.01 vs MMC.
Figure 2.
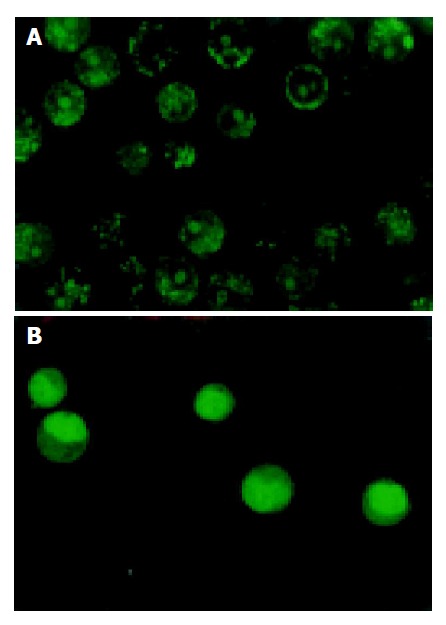
Characteristics of untreated gastric cells. A: and treated gastric cells; B: with drugs.
Effect of drugs on cell cycle phase distribution and apoptosis
The hypodiploid peak before the G1 phase on histogram was called apoptotic cells in FCM analysis. The apoptotic rates of cells incubated in combined drug medium for 24 h were significantly higher than those in MMC treatment group and other groups (P<0.01). The proportion of cells increased in the G0/G1 phase and decreased in the S and G2/M phases of the cell cycle in all three groups.
Expression of COX-2 and Bcl-2 protein
Positive of COX-2 and Bcl-2 expression was found in control group. The positive staining of COX-2 and Bcl-2 in control group showed brown particles, and were distributed mainly in cytoplasm or nuclear membrane and strong positive staining was found in nuclei. Expression of Bcl-2 protein was mainly in membrane and nuclei of cells. The absorbance value (A) of positive cells was detected by image analysis software. The mean value under three random fields was regarded as the relative level of COX-2 and Bcl-2 protein expression. After treatment with drugs for 24 h, the protein level of COX-2 and Bcl-2 decreased in sulindac treatment group and increased in MMC treatment group. After being treated with combination of sulindac and MMC, COX-2 protein level reduced, expression of Bcl-2 remained unchanged as compared to control group, but decreased as compared to MMC treatment group (Table 2).
Table 2.
Expression of COX-2, Bcl-2 protein in SGC-7901 cells after incubated with three drugs for 24 h (mean±SD, n = 3, A).
| Group | COX-2 | Bcl-2 |
| Control | 0.4980±0.0330 | 0.3652±0.057 |
| Sulindac | 0.3967±0.0306a | 0.2617±0.0333b |
| MMC | 0.5633±0.0208 | 0.4500±0.0436b |
| Combined | 0.4000±0.0265ac | 0.3660±0.0397c |
P<0.05,
P<0.01, vs control,
P<0.05 vs MMC.
DISCUSSION
Drug resistance of tumor cells to therapeutic agents derives from multi-mechanism, the abnormal expression of Bcl-2 gene may be involved in it[12]. Recent studies have demonstrated that it can enhance the sensitivity of therapeutic agents and inhibit the expression of Bcl-2[13]. Overexpression of COX-2 has been found in many tumor cells, such as gastric cancer SGC-7901 cells[14]. Several studies demonstrated that elevated expression of COX-2 gene causes up-regulation of Bcl-2 protein and inhibition of cell apoptosis[15]; COX-2 is a upstream regulator of Bcl-2 and may have effect on apoptosis of tumor cell by influencing Bcl-2 gene[16]. Recent studies showed that sulindac has significant anti-proliferation effect on the human gastric adenocarcinoma cell lines MKN45 and MKN28 in vitro and induces apoptosis of cells, and indicating that apoptosis of gastric cells induced by sulindac is involved in the inhibition of COX-2 activity which decreases the level of Bcl-2[7]. Our results in the present study demonstrated that growth inhibition in combined treatment group was more obvious as compared to MMC or sulindac treatment group. FCM showed that the apoptotic rate was higher in combined treatment group than in MMC or sulindac treatment group. After treated with drugs for 24 h, the levels of COX-2 and Bcl-2 protein increased in MMC group, and decreased in combined group, indicating that MMC-induced apoptosis is reduced, which may be caused by up-regulating the expression of COX-2 and Bcl-2 genes, and apoptosis of gastric cancer cells may be mediated by the down-regulation of Bcl-2 and COX-2 gene. A study reported that after exposure of human gastric cancer MKN74 cells to MMC, up-regulation of COX-2 and Bcl-2 protein expression is noted. Furthermore, cotreatment with MMC and NS-398 can down-regulate protein expression and enhance apoptosis of gastric cancer cells[17]. In this study, inhibition of expression of COX-2 and Bcl-2 by sulindac was observed, and Bcl-2 level was much lower in combined group as compared to MMC group.
Cell apoptosis is a active, cellular suicide process regulated by genes, and involves many apoptosis-related genes, such as P53, Bcl-2, C-ntyc. Bcl-2 is one of the final joint pathways of apoptosis[18]. Apoptosis induced by a number of antitumor drugs can be inhibited by Bcl-2[19]. The commonly used antitumor drugs combined with COX-2 inhibitors or antisense gene agents can enhance chemotherapeutic efficacity, reduce drug dose and adverse side effect[19,20]. Our results in the present study indicate that inhibition of proliferation and induction of apoptosis by MMC in combination with sulindac in human gastric cells are enhanced, which may be mediated by the down-regulation of apoptosis-related Bcl-2 gene. To date, however, the exact mechanisms accounting for the signal conductive process between COX-2 and Bcl-2 are still not fully understood. Based on current studies, aside from COX-dependent mechanism, some of them are COX-independent[21-24]. Sulindac and its derivatives have different mechanisms in inducing apoptosis of cancer cells. In conclusion, the combination of MMC and sulindac is more effective on inhibiting cell growth than either MMC or sulindac in cultured SGC-7901 cells.
Footnotes
Co-first-authors: Li Ma and Yong-Le Xie
References
- 1.Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733–1740. [PubMed] [Google Scholar]
- 2.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002;13:669–678. doi: 10.1093/annonc/mdf125. [DOI] [PubMed] [Google Scholar]
- 4.Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506–511. [PubMed] [Google Scholar]
- 5.Fantappiè O, Masini E, Sardi I, Raimondi L, Bani D, Solazzo M, Vannacci A, Mazzanti R. The MDR phenotype is associated with the expression of COX-2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology. 2002;35:843–852. doi: 10.1053/jhep.2002.32469. [DOI] [PubMed] [Google Scholar]
- 6.Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, Kimura A, Komori M, Irie T, Miyoshi E, et al. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567–1572. [PubMed] [Google Scholar]
- 7.Sun B, Wu YL, Zhang XJ, Wang SN, He HY, Qiao MM, Zhang YP, Zhong J. Effects of Sulindac on growth inhibition and apoptosis induction in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:997–1002. [Google Scholar]
- 8.Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, DuBois RN. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001;120:1713–1719. doi: 10.1053/gast.2001.24844. [DOI] [PubMed] [Google Scholar]
- 9.Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 10.Wight NJ, Gottesdiener K, Garlick NM, Atherton CT, Novak S, Gertz BJ, Calder NA, Cote J, Wong P, Dallob A, et al. Rofecoxib, a COX-2 inhibitor, does not inhibit human gastric mucosal prostaglandin production. Gastroenterology. 2001;120:867–873. doi: 10.1053/gast.2001.22432. [DOI] [PubMed] [Google Scholar]
- 11.Zhao LQ. Celecoxib has been approved by FDA in patients with familial adenomatous polyposis. Guowai Yixue Yaoxue Fence. 2000;27:319–320. [Google Scholar]
- 12.Ziegler A, Luedke GH, Fabbro D, Altmann KH, Stahel RA, Zangemeister-Wittke U. Induction of apoptosis in small-cell lung cancer cells by an antisense oligodeoxynucleotide targeting the Bcl-2 coding sequence. J Natl Cancer Inst. 1997;89:1027–1036. doi: 10.1093/jnci/89.14.1027. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZY, Xiao B, Shi YJ, Zhao YQ, Fan DM. Effect transfection of antisense oligonucleotides Bcl-2 on drug sensitivity in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:796–797. [Google Scholar]
- 14.Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 15.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 17.Hsueh CT, Chiu CF, Kelsen DP, Schwartz GK. Selective inhibition of cyclooxygenase-2 enhances mitomycin-C-induced apoptosis. Cancer Chemother Pharmacol. 2000;45:389–396. doi: 10.1007/s002800051007. [DOI] [PubMed] [Google Scholar]
- 18.Chen CJ, Sun YX, Zhou HG, Pan BR, Zheng SG, Hong XZ, Liu JH, Feng WY. bcl-2 and p53 expressions in colorectal adenoma and carcinoma. Shijie huaren Xiaohua Zazhi. 1998;6:683–685. [Google Scholar]
- 19.Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- 20.Wu GS, Wu XY, Zou SQ, Qiu FZ. Effects of cyclooxygenase-2 antisense vector on proliferation of human cholan-giocarcinoma cells. Shijie Huaren Xiaohua Zazhi. 2003;11:733–736. [Google Scholar]
- 21.Yamamoto H, Itoh F, Fukushima H, Hinoda Y, Imai K. Overexpression of cyclooxygenase-2 protein is less frequent in gastric cancers with microsatellite instability. Int J Cancer. 1999;84:400–403. doi: 10.1002/(sici)1097-0215(19990820)84:4<400::aid-ijc12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 23.Gilhooly EM, Rose DP. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int J Oncol. 1999;15:267–270. [PubMed] [Google Scholar]
- 24.Majima M, Hayashi I, Muramatsu M, Katada J, Yamashina S, Katori M. Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants. Br J Pharmacol. 2000;130:641–649. doi: 10.1038/sj.bjp.0703327. [DOI] [PMC free article] [PubMed] [Google Scholar]


