Abstract
AIM: Although an association between hepatic steatosis and vascular risk factors has been described, direct relationships between fatty liver and atherosclerosis have not yet been investigated. The aim of the present study has been to investigate those relationships.
METHODS: The Study of Health in Pomerania examined a random population sample aged between 20 and 79 years. A study population of 4222 subjects without hepatitis B and C infections and without liver cirrhosis was available for the present analysis. Hepatic steatosis was defined sonographically and intima-media thickness (IMT) as well as plaque prevalence were estimated by carotid ultrasound.
RESULTS: The prevalence rate of hepatic steatosis was 29.9%. Among subjects aged ≥45 years, an association between hepatic steatosis and IMT of the carotid arteries was found in bivariate analysis, but not after adjustment for atherosclerotic risk factors. Individuals with fatty liver had more often carotid plaques than persons without fatty liver (plaque prevalence rate 76.8% vs 66.6%; P<0.001). This association persisted after adjustment for confounding factors and was predominantly present in subjects with no to mild alcohol consumption.
CONCLUSION: There is an independent association between hepatic steatosis and carotid atherosclerotic plaques. Metabolic changes due to nonalcoholic fatty liver disease may explain this relationship.
Keywords: Hepatic steatosis, Fatty liver, Atherosclerosis, Study of Health in Pomerania
INTRODUCTION
Fatty liver is a common clinical and histological finding. If there is no co-existing liver disorder such as alcoholic hepatitis or steatohepatitis, further local organ damage does not occur[1,2]. Hepatic steatosis is usually diagnosed by ultrasound with high diagnostic quality. In comparison to histology, liver sonography has been shown to have an 89% diagnostic sensitivity and 93% diagnostic specificity[3].
Fatty liver is associated with several atherosclerotic risk factors such as hypertension, diabetes and dyslipidemia[4,5]. It has also been related to insulin resistance[6-8]. This association was found in NIDDM patients[6] as well as in non-diabetic subjects[7,8]. Although an association between hepatic steatosis and atherosclerotic risk factors has been described, possible direct relationships between hepatic steatosis and atherosclerosis remain to be investigated. The intima-media thickness (IMT) of the carotid artery can be measured non-invasively by ultrasound techniques. An increased IMT has been shown to be a risk factor for myocardial infarction and stroke[9-12]. Furthermore, carotid ultrasound is an accurate diagnostic tool for detecting atherosclerotic plaques and for assessing the degree of luminal narrowing caused by atherosclerotic changes of the vessel wall[13].
The aim of the present study was to investigate associations between hepatic steatosis and the risk of atherosclerosis by analyzing a sample of randomly selected individuals.
MATERIALS AND METHODS
Study population
The Study of Health in Pomerania (SHIP) is a cross-sectional survey in West Pomerania, the north-eastern area of Germany[14]. A random sample from the population aged between 20 and 79 years was drawn. The sample was selected using population registries. Only individuals with German citizenship and main residency in the study area were included. Finally, 7008 subjects were sampled. The net sample (without migrated or deceased persons) comprised 6267 eligible subjects. The SHIP population comprised 4310 participants (68.8% of eligible subjects). Data were collected between October 1997 and May 2001. All participants gave informed written consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in an a priori approval by the local ethics committee.
Of 4310 SHIP participants, 3 refused liver ultrasound examination, 46 had an uncertain diagnosis of hepatic steatosis, 15 tested positive for HBsAg, 21 tested positive for anti-HCV and 8 had a known history of liver cirrhosis. In all, 88 participants were excluded from further analysis. This resulted in a study population of 4222 individuals (2070 men and 2152 women) who were available for the present analysis.
Measurements
Sociodemographic characteristics and medical histories were assessed by computer-aided face-to-face interviews. Alcohol drinking habits were evaluated as beverage-specific alcohol consumption (beer, wine, distilled spirits) on the last weekend and last weekday preceding the examination, and the mean daily alcohol consumption was calculated using beverage-specific pure ethanol volume proportions[15]. Participants were divided into four categories with respect to the mean daily alcohol consumption: (1) none, (2) mild (<20 g alcohol/d), (3) moderate (20-30 g alcohol/d in women, 20-60 g alcohol/d in men), (4) heavy (>30 g alcohol/d in women, >60 g alcohol/d in men). Height and weight were measured for the calculation of the body mass index (BMI = weight [kg]/height2 [m2]). Overweight was defined as a BMI ≥25 kg/m2. Systolic and diastolic blood pressure was measured and hypertension was defined as an average systolic blood pressure ≥140 mmHg, an average diastolic blood pressure ≥90 mmHg, or self-reported use of antihypertensive medication. Diabetes was defined as self-reported physician diagnosis of diabetes, or serum hemoglobin (Hb) A1c >7%.
Non-fasting blood samples were drawn from the cubital vein in the supine position. All analytical laboratories involved in this study participated semi-annually in the official national German tests for quality assurance. In addition, duplicate blood samples were analyzed for internal quality assurance every week. Serum LDL and HDL cholesterol, serum glutamate oxaloacetate transaminase (GOT), glutamic pyruvic transaminase (GPT), and γ-glutamyl transpeptidase (GGT) were measured photometrically (Hitachi 704, Roche, Mannheim, Germany). Lipoprotein (a) concentrations were determined with the use of an immunoluminometric assay (Magic Lite Analyzer II, Ciba Corning, MA, USA). Serum levels of carbohydrate deficient transferrin (CDT) were measured by an immunoassay (Cobas Mira, Roche, Basel, Switzerland). Plasma fibrinogen concentrations were assayed according to Clauss[16] (Electra 1600 analyzer, Instrumentation Laboratory, Barcelona, Spain). The mean corpuscular volume (MCV) of the erythrocytes was determined by measurements of electrical resistance (Coulter Electronics, Hialeah, USA). Markers of hepatitis B virus (HBsAg) and hepatitis C virus infection (anti-HCV) were determined by enzyme-linked immunosorbent assays (AxSym HBSAG and AxSym HCV, Abbot, Abbot Park, USA).
Trained physicians examined the liver using a 5 MHz transducer and a high-resolution instrument (Vingmed VST Gateway, Santa Clara, USA). The sonographers were unaware of the participant’ clinical and laboratory characteristics. Hepatic steatosis was defined as the presence of an ultrasonographic pattern of a bright liver, with evident contrast between hepatic and renal parenchyma[17].
In participants who were ≥45 years, the extracranial carotid arteries were bilaterally examined with high-resolution B-mode ultrasound using a 5 MHz linear array transducer (Diasonics VST Gateway, Santa Clara, USA). Plaques were present if a focal widening of the vessel wall relative to adjacent segments was found (as evident from the protrusion into the lumen and/or localized roughness with increased echogenicity and/or an area of focal increased thickness of the intima-media layer ≥1.3 mm)[18,19]. Plaque prevalence was defined as the presence of one or more plaques in the common carotid artery, the bifurcation, and the internal and external carotid arteries. Scans from the distal straight portion of both common carotid arteries were recorded. Certified readers calculated the mean far-wall IMT by averaging the 10 consecutive measurement points (in 1-mm steps) from the bulb of both sides. The intra-/inter-reader and intra-/inter-observer reliabilities have been published and show Spearman correlation coefficients of >0.9, mean differences <1% and SD of differences <10%[20].
Statistical analysis
Data on quantitative characteristics are expressed as mean±SD. Data on qualitative characteristics are expressed as percentage values or absolute numbers as indicated. Participants were divided into two groups according to the presence or absence of hepatic steatosis. Comparisons between groups were made using ANOVA (continuous data) and χ2-test (nominal data). Multivariate comparisons with respect to the occurrence of plaques were performed by logistic regression and with respect to IMT by analysis of co-variance (ANCOVA). Known risk factors for atherosclerosis and potential confounders such as demographic variables (age, gender), data on risk behavior (smoking and alcohol drinking status) and clinical characteristics (hypertension, diabetes, BMI, total cholesterol/HDL ratio, and plasma fibrinogen concentrations) were included. The odds ratios (OR) were calculated for dichotomized endpoints, values were given with the model-based lower and upper 95% confidence interval (CI). The fit of regression models was evaluated by the adjusted R2. A value of P<0.05 was considered statistically significant. All statistical analyses were performed with SPSS software, version 11.0.1 (SPSS GmbH Software, Munich, Germany).
RESULTS
Hepatic steatosis was diagnosed in 1261 of the 4222 participants (prevalence rate 29.9%). Persons with and without hepatic steatosis were compared with respect to age, gender, alcohol consumption and serological liver characteristics (Table 1). Individuals with fatty liver were older, more often of male gender and reported higher alcohol consumption than individuals without hepatic steatosis. Furthermore, they had higher MCV values and higher serum concentrations of GGT, GOT and GPT. Persons with and without hepatic steatosis did not differ with respect to serum CDT levels.
Table 1.
Baseline characteristics of persons with and without hepatic steatosis.
| Unit | No teatosis n = 2 961 | Steatosis n = 1 261 | P | |
| Age | yr | 47.1±16.7 | 57.2±13.3 | <0.001 |
| Gender (male) | 1283 (43.3%) | 787 (62.4%) | <0.001 | |
| Mean daily alcohol | mg/d | 10.6±16.2 | 16.0±23.1 | <0.001 |
| consumption | ||||
| Alcohol consumption | ||||
| none | 1060 (35.8%) | 415 (32.9%) | <0.001 | |
| mild | 1331 (45.0%) | 477 (37.8%) | ||
| moderate | 258 (8.7%) | 129 (10.2%) | ||
| heavy | 294 (9.9%) | 234 (18.6%) | ||
| MCV | mm³ | 89.9±4.4 | 90.6±4.8 | <0.001 |
| CDT | % | 4.8±1.6 | 4.9±1.8 | 0.23 |
| GGT | mmol/L×s | 0.4±0.4 | 1.0±2.2 | <0.001 |
| GOT | mmol/L×s | 0.3±0.1 | 0.5±0.3 | <0.001 |
| GPT | mmol/L×s | 0.4±0.2 | 0.7±0.4 | <0.001 |
χ2 test (nominal data) or ANOVA (interval data). MCV, mean corpuscular volume; CDT, carbohydrate-deficient transferrin; GGT, γ-glutamyl transpeptidase; GOT, glutamate oxaloacetate transaminase; GPT, glutamic pyruvic transaminase.
Among the atherosclerotic risk factors, persons with hepatic steatosis more often had diabetes, hypertension and overweight than those without hepatic steatosis (Table 2). Furthermore, individuals with hepatic steatosis had higher values for systolic as well as diastolic blood pressure, higher BMI, higher serum HbA1c, cholesterol and LDL cholesterol levels and lower HDL cholesterol levels. Differences between both groups with respect to smoking habits and plasma fibrinogen levels failed to attain statistical significance after adjustment for age and gender. Individuals with and without hepatic steatosis did not differ with respect to serum lipoprotein (a) levels.
Table 2.
Atherosclerotic risk factors in persons with and without hepatic steatosis.
| Unit |
No steatosis |
Steatosis |
P1 (unadjusted) | P2 (adjusted) | |
| n = 2961 | n = 1261 | ||||
| Smoking (current) | 984 (33.2%) | 335 (26.7%) | <0.001 | 0.75 | |
| Hemoglobin A1c | % | 5.3±0.8 | 5.9±1.2 | <0.001 | <0.001 |
| Diabetes mellitus | 134 (4.5%) | 240 (19.0%) | <0.001 | <0.001 | |
| Systolic blood pressure | mmHg | 133±21 | 146±20 | <0.001 | <0.001 |
| Diastolic blood pressure | mmHg | 82±11 | 87±11 | <0.001 | <0.001 |
| Hypertension | 1274 (43.0%) | 945 (74.9%) | <0.001 | <0.001 | |
| BMI | kg/m² | 26.1±4.3 | 30.1±4.6 | <0.001 | <0.001 |
| Overweight | 1657 (56.0%) | 1116 (88.5%) | <0.001 | <0.001 | |
| Cholesterol | mmol/L | 5.7±1.2 | 6.1±1.3 | <0.001 | <0.001 |
| LDL cholesterol | mmol/L | 3.5±1.2 | 3.8±1.2 | <0.001 | 0.01 |
| HDL cholesterol | mmol/L | 1.5±0.4 | 1.3±0.5 | <0.001 | <0.001 |
| Lipoprotein (a) | mg/L | 217±283 | 204±280 | 0.15 | 0.06 |
| Fibrinogen | g/L | 2.9±0.7 | 3.1±0.7 | <0.001 | 0.26 |
1χ2-test (nominal data) or ANOVA (interval data). 2Logistic regression (nominal data) or analysis of covariance (interval data), adjusted for gender and age. BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Analyses of the association between hepatic steatosis and IMT and the occurrence of plaques were performed in the data set of 2432 participants aged 45 years or older (992 with steatosis and 1440 without). Persons with hepatic steatosis had higher IMT values than persons without steatosis (Table 3). The strength of this association was attenuated after adjustment for age and gender and did not attain statistical significance after adjustment for the full model (Table 3). The full regression model explained 25.6% of the variability of carotid IMT.
Table 3.
Carotid IMT and plaques in persons with and without hepatic steatosis.
| Unit | No steatosis | Steatosis | P | |
| IMT | mm | 0.784±0.172 | 0.811±0.170 | <0.001 |
| IMT, adjusted for gender and age | mm | 0.789±0.004 | 0.803±0.005 | <0.05 |
| IMT, adjusted for the full model1 | mm | 0.813±0.004 | 0.806±0.005 | 0.34 |
| Plaque | OR (CI) | 1.0 (Ref.) | 1.66 (1.38–2.00) | <0.001 |
| Plaque, adjusted for gender and age | OR (CI) | 1.0 (Ref.) | 1.60 (1.30–1.97) | <0.001 |
| Plaque, adjusted for full model1 | OR (CI) | 1.0 (Ref.) | 1.35 (1.03–1.61) | <0.05 |
Full model included gender, age, alcohol consumption, current smoking, diabetes, hypertension, BMI, total cholesterol/HDL ratio, plasma fibrinogen levels. IMT, carotid intima-media thickness; OR, odds ratio; CI, 95% confidence interval.
The overall prevalence rate of atherosclerotic carotid plaque was 70.8%. Seven hundred and sixty-two individuals with hepatic steatosis (76.8%) had at least one carotid plaque compared to 959 subjects without steatosis (66.6%; P<0.001). Age-stratified analyses revealed higher plaque prevalence in persons with fatty liver in all age decades except the oldest (Figure 1). The association between hepatic steatosis and carotid plaques remained statistically significant after adjustment for age and gender as well as for known atherosclerotic risk factors and possible confounders (Table 3) and also if the ultrasound operator was included as a co-factor. The regression model explained 31.9% of the variability of plaque.
Figure 1.
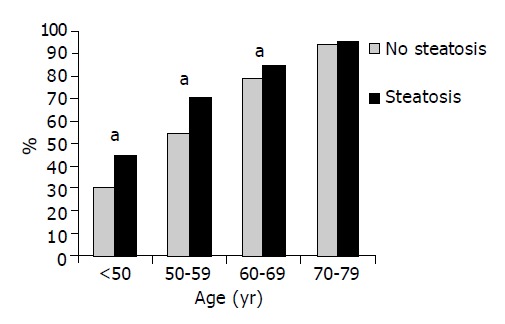
The prevalence of carotid plaques with respect to the absence and presence of hepatic steatosis. aP<0.05 vs Steatosis.
Multivariable analyses were repeated after the study population was dichotomized by the mean daily alcohol consumption. Among the individuals with no to mild alcohol use (<20 g per d), subjects with hepatic steatosis had an increased risk of carotid atherosclerotic plaques (adjusted OR for steatosis 1.31; 95%CI 1.01-1.71; P<0.05), whereas this association was not present in the subpopulation of subjects with a mean daily alcohol consumption of 20 g or more (adjusted OR for steatosis 1.07; 95%CI 0.62-1.83; P = 0.81). An independent relationship between hepatic steatosis and carotid IMT was not found in any of the sub-populations.
DISCUSSION
Possible associations between hepatic steatosis and atherosclerosis were systematically studied using different endpoints. Our study showed an association between hepatic steatosis and prevalent atherosclerotic plaques of the carotid artery. This relationship remained statistically significant after adjustment for age and gender as well as for possible confounders and atherogenic risk factors. An independent association between fatty liver and IMT was not observed. To the best of our knowledge, this is the first study which could demonstrate an independent relationship between hepatic steatosis and the risk of carotid atherosclerosis.
Apart from moderate alcohol intake which may protect against atherosclerosis[21], most of the established atherosclerotic risk factors such as diabetes, hypertension, obesity and serum lipid disturbances were more prevalent in subjects with hepatic steatosis. This is in concordance with previous studies[4,5,7,17]. Thus, it might be assumed that the association between hepatic steatosis and atherosclerotic plaques was due to a paralleled distribution of those risk factors. However, it is not clear whether hepatic steatosis plays an active role in the development and progression of especially metabolic risk factors. For example, an association between diabetes and fatty liver has been described[4], but it remains to be seen whether hepatic steatosis is indeed a causal factor for diabetes or is simply a consequence of the disease. Recently, healthy individuals with fatty liver were shown to have increased fasting plasma insulin concentrations and a decreased insulin clearance[7]. This suggests that hepatic steatosis may cause or promote insulin resistance early in a pre-diabetic state. These findings are consistent with data collected from individuals with nonalcoholic fatty liver disease showing an association between hepatic steatosis and insulin resistance even in lean subjects with normal glucose tolerance[22,23]. Findings from other studies[24,25] suggest that nonalcoholic fatty liver disease is another feature of the metabolic syndrome.
Further insight into the pathobiological role of hepatic steatosis in this context is provided by studies that used serum GGT, a good marker for hepatic steatosis[4,5], as the exposure variable and that investigated certain metabolic endpoints. Data from a large prospective study[26] demonstrated that elevated serum GGT levels at baseline predicted incident NIDDM. This relationship was independent of other NIDDM risk factors such as obesity and physical activity and remained stable after adjustment for alcohol consumption. Other studies[27,28] have found a relationship between serum GGT levels and cardiovascular risk factors such as hypertension, BMI or dyslipidemia. Together with the findings of our study, those data suggest that hepatic steatosis may not only be a morphological characteristic, but also has functional importance in the development of metabolic atherosclerotic risk factors and atherosclerosis itself.
Some associations between hepatic steatosis and disorders such as diabetes and the metabolic syndrome were found to be present in subjects with nonalcoholic fatty liver disease[22-25]. In the present study, the relationship between hepatic steatosis and carotid atherosclerotic plaque was found in the whole population and was free from alcohol consumption. However, further analyses revealed that this association was only present in subjects with mean daily alcohol consumption of <20 g. This finding therefore supports the concept of nonalcoholic fatty liver disease.
After adjustment for the full risk factor model in the whole study population, persons with hepatic steatosis had similar IMT values compared to those without. This finding may appear contradictory, because on the one hand we found an association between hepatic steatosis and prevalent atherosclerotic plaques, but on the other hand IMT is known to be a marker for generalized atherosclerosis[9-11]. However, it should be considered that IMT is only a marker for atherosclerosis and not atherosclerosis itself. Mechanisms and disorders that cause hypertrophic changes of artery vessel walls may increase IMT without elevating the risk of atherosclerosis. For example, it has been shown that treatment with triodothyronine causes hypertrophy of coronary arteries[29]. This indicates thicker artery walls in hyperthyroidism, whereas hypothyroidism is known to cause atherosclerosis[30]. Another example is that plaque characteristics but not IMT were independently associated with incident coronary events among patients with coronary artery disease[31]. Thus, the association between fatty liver and IMT which we found by bivariate testing was substantially confounded by other factors.
In agreement with other studies[4,32] we have found an association between hepatic steatosis and male gender. Also in line with other studies[17], our data further obtained a relationship between alcohol consumption and hepatic steatosis. However, one Japanese study[4] did not find such association. This may be due to ethnic differences. Unfortunately, questions on drinking habits in the Japanese study[4] did not refer to the time of ultrasound investigation, but to some years before, thereby limiting the comparability of their results. In our study the relationship between alcohol consumption and hepatic steatosis was not reflected by an association between fatty liver and serum CDT levels. This may be explained by the limited diagnostic value of CDT for the evaluation of problematic alcohol consumption which is currently undergoing debate[33].
Some limitations of this study merit consideration. Firstly, data were derived from a cross-sectional study, so that we can only hypothesize a causal effect of hepatic steatosis on carotid atherosclerosis. Secondly, intra- and inter-observer agreements with respect to the diagnosis of hepatic steatosis were not measured. Therefore, we repeated all multivariable analyses with the observer as a co-factor in order to control, for a possible operator-bias. These additional analyses revealed similar results concerning the association between fatty liver and the investigated endpoints. Additionally, the expected pattern of risk factors for hepatic steatosis argues against a low accuracy and validity in the diagnosis of this disorder. Thirdly, ultrasound may not be the method of choice to distinguish fat from fibrosis or early cirrhosis. A biopsy as golden standard for diagnosing hepatic steatosis was not practicable for this population-based study and was reserved for patients with clinically relevant liver disease. However, if in our study some of the participants with early cirrhosis were misclassified as having steatosis, we would have consequently underestimated the risk of atherosclerosis in individuals with fatty liver because of the protective effects of liver cirrhosis against atherosclerotic processes[34]. Finally, we could only analyze non-fasting blood samples for practical reasons. The participants were non-fasting due to the duration of the cumulative examinations, 4-6 h in total. Therefore, serum triglycerides and glucose were not included in the present analysis.
We conclude that there is an association between hepatic steatosis and carotid atherosclerosis which is free from other atherogenic risk factors and predominantly present in subjects with abstinence to mild alcohol consumption. Metabolic changes due to nonalcoholic fatty liver disease may contribute to this relationship.
ACKNOWLEDGEMENTS
The work is part of the Community Medicine Research net (CMR) of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grant no. ZZ9603), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania.
Footnotes
Supported by Community Medicine Research net (CMR) of the University of Greifswald, which is funded by the Federal Ministry of Education and Research and the Federal State of Mecklenburg-West Pomerania
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 2.Evans CD, Oien KA, MacSween RN, Mills PR. Non-alcoholic steatohepatitis: a common cause of progressive chronic liver injury? J Clin Pathol. 2002;55:689–692. doi: 10.1136/jcp.55.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- 4.Akahoshi M, Amasaki Y, Soda M, Tominaga T, Ichimaru S, Nakashima E, Seto S, Yano K. Correlation between fatty liver and coronary risk factors: a population study of elderly men and women in Nagasaki, Japan. Hypertens Res. 2001;24:337–343. doi: 10.1291/hypres.24.337. [DOI] [PubMed] [Google Scholar]
- 5.Osono Y, Nakajima K, Hata Y. Hypertriglyceridemia and fatty liver: clinical diagnosis of fatty liver and lipoprotein profiles in hypertriglyceridemic patients with fatty liver. J Atheroscler Thromb. 1995;2 Suppl 1:S47–S52. doi: 10.5551/jat1994.2.supplement1_s47. [DOI] [PubMed] [Google Scholar]
- 6.Banerji MA, Buckley MC, Chaiken RL, Gordon D, Lebovitz HE, Kral JG. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obes Relat Metab Disord. 1995;19:846–850. [PubMed] [Google Scholar]
- 7.Goto T, Onuma T, Takebe K, Kral JG. The influence of fatty liver on insulin clearance and insulin resistance in non-diabetic Japanese subjects. Int J Obes Relat Metab Disord. 1995;19:841–845. [PubMed] [Google Scholar]
- 8.Ikai E, Ishizaki M, Suzuki Y, Ishida M, Noborizaka Y, Yamada Y. Association between hepatic steatosis, insulin resistance and hyperinsulinaemia as related to hypertension in alcohol consumers and obese people. J Hum Hypertens. 1995;9:101–105. [PubMed] [Google Scholar]
- 9.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 10.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 11.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 12.Bots ML, Hoes AW, Hofman A, Witteman JC, Grobbee DE. Cross-sectionally assessed carotid intima-media thickness relates to long-term risk of stroke, coronary heart disease and death as estimated by available risk functions. J Intern Med. 1999;245:269–276. doi: 10.1046/j.1365-2796.1999.0442f.x. [DOI] [PubMed] [Google Scholar]
- 13.Long A, Lepoutre A, Corbillon E, Branchereau A. Critical review of non- or minimally invasive methods (duplex ultrasonography, MR- and CT-angiography) for evaluating stenosis of the proximal internal carotid artery. Eur J Vasc Endovasc Surg. 2002;24:43–52. doi: 10.1053/ejvs.2002.1666. [DOI] [PubMed] [Google Scholar]
- 14.John U, Greiner B, Hensel E, Lüdemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, et al. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Soz Praventivmed. 2001;46:186–194. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- 15.Alte D, Lüdemann J, Piek M, Adam C, Rose HJ, John U. Distribution and dose response of laboratory markers to alcohol consumption in a general population: results of the study of health in Pomerania (SHIP) J Stud Alcohol. 2003;64:75–82. doi: 10.15288/jsa.2003.64.75. [DOI] [PubMed] [Google Scholar]
- 16.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 17.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 18.Gray-Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino) 1988;29:676–681. [PubMed] [Google Scholar]
- 19.Auperin A, Berr C, Bonithon-Kopp C, Touboul PJ, Ruelland I, Ducimetiere P, Alperovitch A. Ultrasonographic assessment of carotid wall characteristics and cognitive functions in a community sample of 59- to 71-year-olds. The EVA Study Group. Stroke. 1996;27:1290–1295. doi: 10.1161/01.str.27.8.1290. [DOI] [PubMed] [Google Scholar]
- 20.Luedemann J, Schminke U, Berger K, Piek M, Willich SN, Döring A, John U, Kessler C. Association between behavior-dependent cardiovascular risk factors and asymptomatic carotid atherosclerosis in a general population. Stroke. 2002;33:2929–2935. doi: 10.1161/01.str.0000038422.57919.7f. [DOI] [PubMed] [Google Scholar]
- 21.Grønbaek M. Factors influencing the relation between alcohol and mortality--with focus on wine. J Intern Med. 2001;250:291–308. [PubMed] [Google Scholar]
- 22.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 23.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 24.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Stravitz RT, Mills AS. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064–2071. doi: 10.1111/j.1572-0241.2003.07640.x. [DOI] [PubMed] [Google Scholar]
- 26.Perry IJ, Wannamethee SG, Shaper AG. Prospective study of serum gamma-glutamyltransferase and risk of NIDDM. Diabetes Care. 1998;21:732–737. doi: 10.2337/diacare.21.5.732. [DOI] [PubMed] [Google Scholar]
- 27.Robinson D, Whitehead TP. Effect of body mass and other factors on serum liver enzyme levels in men attending for well population screening. Ann Clin Biochem. 1989;26(Pt 5):393–400. doi: 10.1177/000456328902600503. [DOI] [PubMed] [Google Scholar]
- 28.Nilssen O, Førde OH, Brenn T. The Tromsø Study. Distribution and population determinants of gamma-glutamyltransferase. Am J Epidemiol. 1990;132:318–326. doi: 10.1093/oxfordjournals.aje.a115661. [DOI] [PubMed] [Google Scholar]
- 29.Sernia C, Marchant C, Brown L, Hoey A. Cardiac angiotensin receptors in experimental hyperthyroidism in dogs. Cardiovasc Res. 1993;27:423–428. doi: 10.1093/cvr/27.3.423. [DOI] [PubMed] [Google Scholar]
- 30.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 31.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol. 2003;42:1037–1043. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 32.Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol. 2001;35:531–537. doi: 10.1016/s0168-8278(01)00151-9. [DOI] [PubMed] [Google Scholar]
- 33.Scouller K, Conigrave KM, Macaskill P, Irwig L, Whitfield JB. Should we use carbohydrate-deficient transferrin instead of gamma-glutamyltransferase for detecting problem drinkers? A systematic review and metaanalysis. Clin Chem. 2000;46:1894–1902. [PubMed] [Google Scholar]
- 34.Marchesini G, Ronchi M, Forlani G, Bugianesi E, Bianchi G, Fabbri A, Zoli M, Melchionda N. Cardiovascular disease in cirrhosis--a point-prevalence study in relation to glucose tolerance. Am J Gastroenterol. 1999;94:655–662. doi: 10.1111/j.1572-0241.1999.00931.x. [DOI] [PubMed] [Google Scholar]


