Abstract
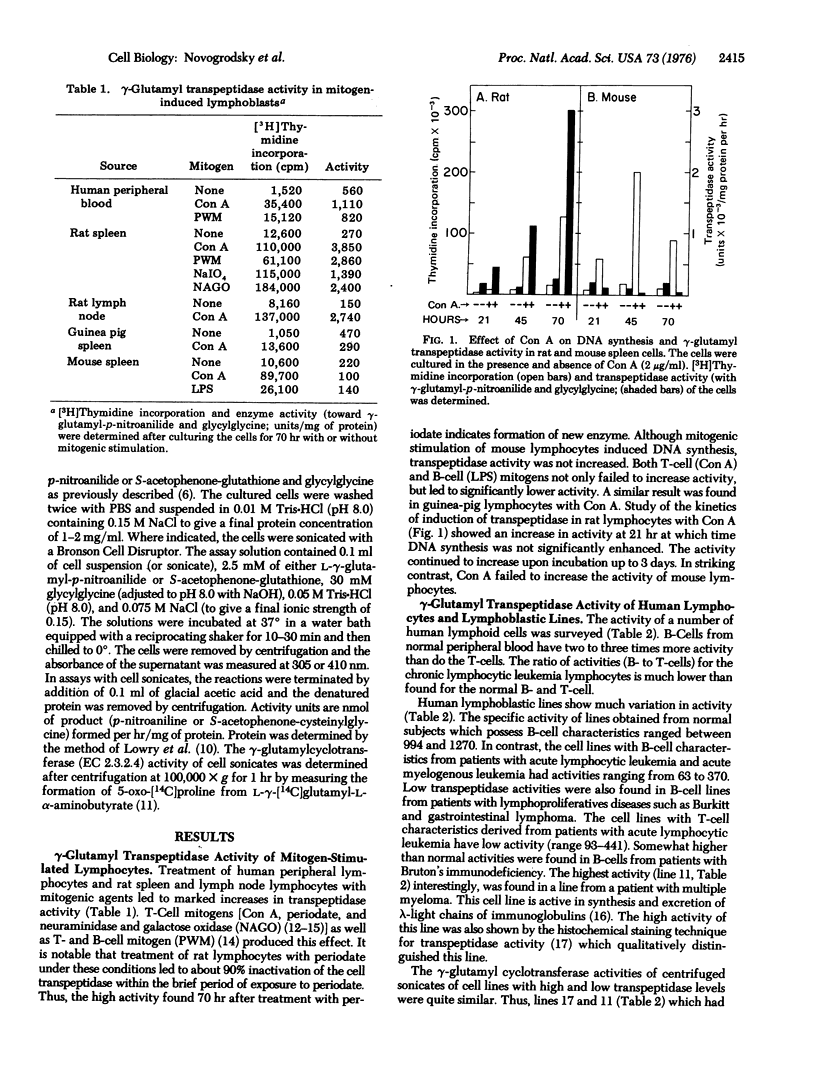
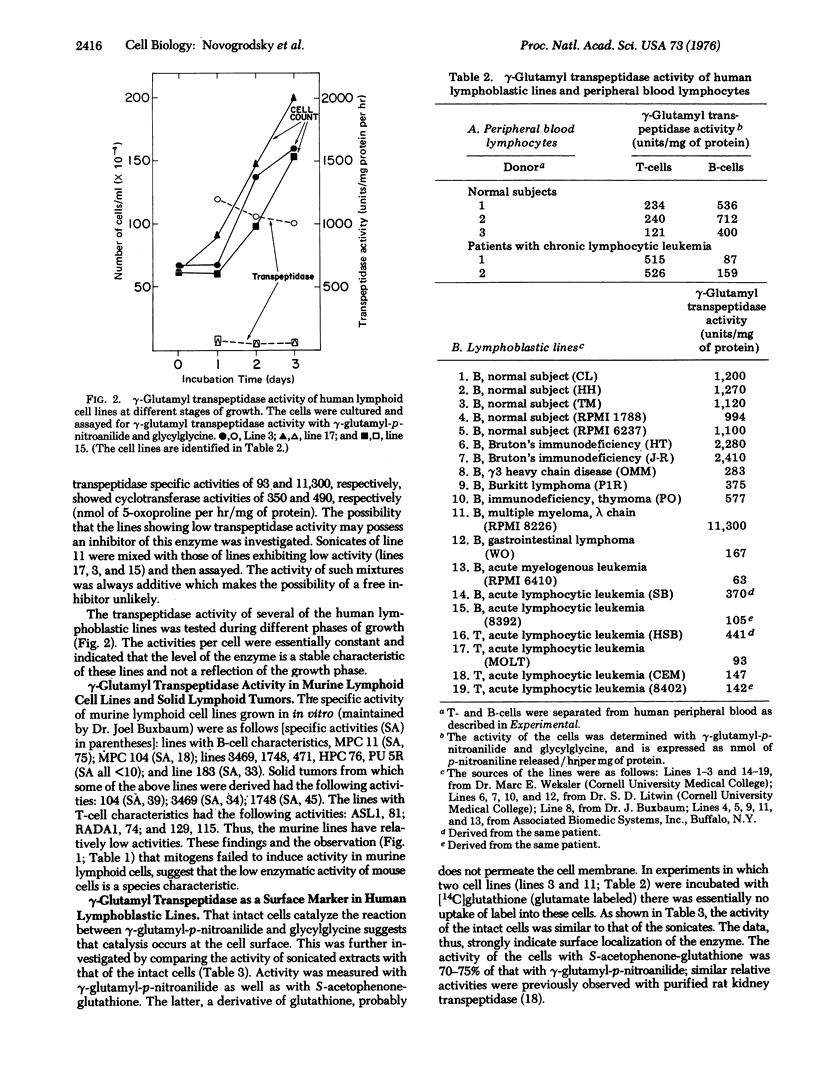
gamma-Glutamyl transpeptidase, an enzyme that catalyzes gamma-glutamyl transfer from gamma-glutamyl compounds to amino acid and peptide acceptors, and which is known to be localized in the membranes of many epithelial cells, was found in a variety of lymphoid cells. The lymphoid cell enzyme is located on the cell surface, and exhibits substantially the same substrate specificity as the enzyme found in epithelial cells. Human and rat (but not mouse) lymphocyte gamma-glutamyl transpeptidase was stimulated by treatment of the cells with mitogens. Normal human peripheral B-cells were more active than T-cells, but the reverse relationship of activities was found in chronic lymphocytic leukemia lymphocytes. Human lymphoblastic lines vary markedly in activity. In general, cell lines with B- and T-characteristics from patients with lymphoproliferative diseases had much lower activities than those of B-cell lines derived from normal subjects. The highest activity found was in a human myeloma line active in synthesis of an immunoglobulin light chain. The data indicate that gamma-glutamyl transpeptidase is a surface marker reflecting differentiation in normal and neoplastic cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litwin S. D., Hütteroth T. H., Lin P. K., Kennard J., Cleve H. Immunoglobulin expression of cells from human lymphoblastoid lines. II. Interrelationship among surface, cellular, and secreted immunoglobulins. J Immunol. 1974 Aug;113(2):668–672. [PubMed] [Google Scholar]
- Matsuoka Y., Moore G. E., Yagi Y., Pressman D. Production of free light chains of immunoglobulin by a hematopoietic cell line derived from a patient with multiple myeloma. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1246–1250. doi: 10.3181/00379727-125-32327. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974 Jul 15;15(2):177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- Meister A. On the enzymology of amino acid transport. Science. 1973 Apr 6;180(4081):33–39. doi: 10.1126/science.180.4081.33. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Induction of lymphocyte transformation by sequential treatment with neuraminidase and galactose oxidase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1824–1827. doi: 10.1073/pnas.70.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Katchalski E. Membrane site modified on induction of the transformation of lymphocytes by periodate. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3207–3210. doi: 10.1073/pnas.69.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Lotan R., Ravid A., Sharon N. Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J Immunol. 1975 Nov;115(5):1243–1248. [PubMed] [Google Scholar]
- Novogrodsky A. Selective activation of mouse T and B lymphocytes by periodate, galactose oxidase and soybean agglutinin. Eur J Immunol. 1974 Oct;4(10):646–648. doi: 10.1002/eji.1830041003. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Richman P. G., Meister A. Isolation and properties of gamma-L-glutamylcyclotransferase from human brain. Biochemistry. 1969 Mar;8(3):1048–1055. doi: 10.1021/bi00831a036. [DOI] [PubMed] [Google Scholar]
- Pick E., Turk J. L. The biological activities of soluble lymphocyte products. Clin Exp Immunol. 1972 Jan;10(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- REVEL J. P., BALL E. G. The reaction of glutathione with amino acids and related compounds as catalyzed by gamma-glutamyl transpeptidase. J Biol Chem. 1959 Mar;234(3):577–582. [PubMed] [Google Scholar]
- Ross L. L., Barber L., Tate S. S., Meister A. Enzymes of the gamma-glutamyl cycle in the ciliary body and lens. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2211–2214. doi: 10.1073/pnas.70.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann M., Preud'Homme J. L., Brouet J. C. B and T cell markers in human proliferative blood diseases and primary immunodeficiencies, with special reference to membrane bound immunoglobulins. Transplant Rev. 1973;16:85–113. doi: 10.1111/j.1600-065x.1973.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Identity of maleate-stimulated glutaminase with gamma-glutamyl transpeptidase in rat kidney. J Biol Chem. 1975 Jun 25;250(12):4619–4627. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem. 1974 Dec 10;249(23):7593–7602. [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]



