Abstract
AIM: To determine whether local antibiotic resistance involves P-glycoprotein (Pgp)-mediated active drug out-pumping during Helicobacter pylori (H pylori) infection treatment with classic antibiotic therapy.
METHODS: Pgp activity was determined in gastric mucosa biopsy specimens obtained from 53 patients with pathohistologically verified gastritis and microbiologically confirmed H pylori infection, and compared with the Pgp activity in 12 control subjects with normal endoscopic findings. The H pylori positive patients were treated with short-term 7-d therapy consisting of two antibiotics (amoxicillin and azithromycin/metronidazole and clarithromycin) and a proton pump inhibitor. Pgp activity was determined by flow cytometry in the test of rhodamine dye efflux and quantified as mean fluorescence ratio (RMF).
RESULTS: Upon the first cycle, H pylori was successfully eradicated in 20 patients, whereas therapy was continued in 33 patients. In the course of antibiotic therapy, RMF increased (P<0.05) and gastric cells showed higher rhodamine dye efflux. The mean pre-treatment RMF values were also higher (P<0.0001) in patients with multiple therapeutic failure than in those with successful H pylori eradication and control subjects.
CONCLUSION: Pgp might be one of the causes of therapy failure in patients with H pylori and antibiotic therapy could be chosen and followed up on the basis of the Pgp transporter local activity.
Keywords: H pylori, RMF, Pgp
INTRODUCTION
Protocols that include a small number of antibiotics (metronidazole, tetracycline, amoxicillin, clarithromycin and azithromycin) and other drugs with antimicrobial effect (bismuth and proton pump inhibitors, PPI) are used in the management of Helicobacter pylori (H pylori) infection. According to the Maastricht Consensus, successful treatment of H pylori infection implies a combination of two antibiotics and one antisecretory drug[1,2]. At present, H pylori infection is successfully treated in about 90% of cases; however, 10% of patients remain H pylori positive. Successful H pylori eradication depends on numerous factors. Better success is achieved if there is coexistence of antral gastritis or gastritis of the gastric body[3], and in patients with peptic ulcer infected with H pylori cagA and vacAs1 positive strains than in those with functional dyspepsia[4-6]. The efficacy of H pylori eradication has been shown to be influenced by gastric pH[7], geographic location, and patient compliance during treatment[8]. It should be noted that the development of primary and secondary bacterial resistance depends on the length of treatment, combination of antibiotics, and drug concentration[9,10]. H pylori has developed resistance to many drugs, e.g., metronidazole, clarithromycin, etc.[5,8,11]. Treatment failure has been ascribed to metabolic changes in H pylori itself[5,12]. Repeat drug administration at a higher concentration has been demonstrated to produce better result[7,10,12], suggesting that local conditions are responsible for therapeutic success[8,12].
It is known that a high concentration of P-glycoprotein (Pgp), a multidrug transporter that actively pumps out all potentially cytotoxic substances, is found on the gastrointestinal tract epithelium. Multidrug transporters are transmembranously located, their mechanism of action being based on binding a broad spectrum of drugs to the membrane polypeptide chain and extruding them to the surroundings by use of ATP energy. All ATP dependent efflux proteins belong to the large ABC superfamily[13-15] and are homologous in many animal species[16]. Although the efflux as a mechanism of antibiotic resistance was demonstrated for gram-negative bacteria in the early 1980s[17], the antimicrobial efflux transporter for H pylori, LmrA, homologous to human multidrug transporter has only recently been described[18-20].
MATERIALS AND METHODS
Materials
Gastric biopsy specimens (GBS) were collected from 65 subjects upon their informed consent in writing, according to the Helsinki Declaration and approved by the Hospital Ethics Committee. Fifty-three patients (24 male and 29 female), mean age 47.4 years (range 18-75 years), with endoscopically and pathohistologically verified antral gastritis had H pylori infection and were treated as outpatients at the University Department of Medicine, Sveti Duh General Hospital in Zagreb.
Methods
H pylori was verified by two parallel tests, i.e. microbiology and pathohistology. Microbiologic diagnosis included isolation and typing, whereas antibiotic sensitivity was determined by E test (B-biodisk) and Chaves’ method[21-23]. Histologic diagnosis included material staining according to Giemsa[24]. Control group had 12 patients with dyspepsia as an indication for upper gastrointestinal endoscopy, in whom H pylori infection was neither microbiologically nor histologically demonstrated[21-24].
Treatment of H pylori infection As the study was not aimed at comparison of different therapeutic protocols, it included a higher proportion of patients with multiple therapeutic failure than would otherwise be expected according to the efficacy of H pylori eradication. All patients underwent standard short-term triple therapy consisting of pantoprazole (P), a PPI, at a dose of 40 mg b.i.d., and two antibiotics, according to Maastricht recommendations[1,2,9]. The following antibiotics were used: metronidazole (M), 500 mg; amoxicillin (A), 1 000 mg; and clarithromycin (C), 500 mg b.i.d. for 7 d, in randomized combinations. The patients were randomly allocated to the PAM (n = 39) or PAC (n = 14) protocol. Following initial therapeutic failure in eradication of H pylori infection, the patients were administered a new combination of the listed antibiotics for 10 d, or azithromycin (AZ) at a dose of 500 mg s.i.d. for 5 d in addition to one of the listed antibiotics for 10 d[1,2,25]. So, in the second course after initial failure with the PAM protocol, there were 15 patients in the PAC protocol and PAAZ protocol each. Also, in the second course after initial failure with the PAC protocol, there were three patients on the PAAZ protocol[1,2,9,25]. Following second failure of H pylori eradication, a third combination was introduced, consisting of P 40 mg b.i.d., in combination with bismuth citrate (B) t.i.d., M 500 mg s.i.d., and tetracycline (T) 500 mg q.i.d. for 14 d[1,2,25]. This final protocol included 22 patients. The treatment with pantoprazole, 40 g/d, was continued for another 6 wk after each of these protocols[1,2,9,25]. H pylori eradication was simultaneously assessed by microbiology and pathohistology 7 wk of the completion of each protocol[1,2,9]. Patients who had been receiving anti-inflammatory or MDR-dependent drugs (that may have influenced the efflux) within one year preceding the study were not included[13-20].
Collection of biopsy specimens GBS for microbiology and histology as well as for the assessment of Pgp activity were obtained from the oxyntic area during gastrointestinal endoscopy using an Olympus Q20 and Pentax endoscopy video system, in line with recommendations of the Sydney classification[24]. Two specimens from the antrum and body of the stomach each, and one from the angulus were obtained for microbiologic and histologic analysis, whereas two specimens from the anterior and posterior wall of the antrum each were obtained for the study of Pgp activity.
Microbiologic and histologic analysis Density of intraepithelial neutrophils (DIEn) and H pylori density (DHP) were determined in biopsy specimens as sensitive parameters of mucosal lesion and H pylori presence[24]. H pylori and neutrophil density was scored 1-4 according to Sydney classification.
Determination of Pgp activity. Reagents Rhodamine-123 (Rh123, 2-6-amino 3 imino 3H xanthene 9 y benzoic acid methyl ester, Sigma, St. Louis, MO, USA) is a cationic lipophilic fluorescent dye frequently used to study Pgp activity. The cells were labeled with 1:100 of stock solution in PBS, a final concentration 20 μg/mL. Non-toxic substrates for Pgp, a fungal metabolite Cyclosporine A (CsA, from Tolypocladium inflantum, Sandoz Pharmaceuticals, Switzerland) and homoharringtonine (HHT, an alkaloid from Cephalotaxus hainanensis, Sigma) were used in a final concentration of 2.5 and 150 ng/mL, respectively.
Cell lines For determination of Pgp activity, exponentially growing human erythroleukemic K562 cell line with an optimal density of 5×105/mL were prepared as control cells. The cell lines were grown in an RPMI 1640 nutrient mixture supplemented with 2 μmol/L L-glutamine, 1 μmol/L sodium pyruvate, 1000 units/mL penicillin, 100 mg/mL streptomycin, and 10% fetal calf serum at 37 °C in a humidified atmosphere of 50 mL/L CO2, in air[26]. Negative control: drug-sensitive K562 line was purchased from the ATCC (American Type Culture Collection, Rockville, MD, USA). Positive control: the resistant HHT/K562 cell line obtained by continuous exposure to HHT was a kind gift from Professor J.P. Marie (Hotel Dieu, France) and was cultured in the presence of 150 ng/mL of HHT.
Preparation of GBS Upon endoscopy, the GBS were immediately placed in transport media and then into transport containers with ice at a constant temperature of 4 °C. GBS was dissociated mechanically by mincing the sample with little scissors, then washed with cold buffer and centrifuged at 3000 r/min for 5 min at 4 °C. The viability and yield were assessed by trypan blue exclusion.
Rhodamine efflux measurement A direct functional assay for Pgp activity was performed by the Rh123 uptake/retention method[27] adapted for stomach tissue analysis. Briefly, aliquoted GBS (5-8×105 cells/tube) were stained for 60 min with 5 μL of Rh123 in the presence or absence of MDR-reversing agent CsA (5 μL). After Rh123 uptake, cells were washed and fed with Rh123-free culture medium, cultured for 60 min at 37 °C, again in the presence or absence of CsA to evaluate its effect on Rh123 efflux (retention). Afterwards, GBS was centrifuged in culture medium, resuspended and kept in ice and darkness for 10 min until flow cytometry analysis. As a reference, K562 and HHT 150/K562 cells were processed in parallel with the patient samples. The analysis was performed on an FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) equipped with an ultraviolet argon laser (excitation at 488 nm, emission at 530/30 and 570/30 nm band-pass filters). Analysis of 104 cells per sample was carried out in log histogram and is illustrated in Figure 1. When Rh123 was being diffused into the cell, Pgp actively pumped out the fluorochrome, and cellular fluorescence was determined by the rates of influx and efflux. If another compound, a substrate and/or inhibitor, was presented in the same manner together with Rh123, the efflux was blocked and the fluorescent dye accumulated in the cell, producing higher mean fluorescence intensity. The result can be quantitatively analyzed and expressed as the ratio of the two mean fluorescences. RMF represents the ratio of MF of Rh123 with CsA modulator divided by MF of Rh123 without modulator after subtraction of the fluorescence of the blanks (autofluorescence of cells <1%). As the amount of intracellular Rh123 dye content upon the addition of modulator correlated with Pgp activity, RMF ≥1 was considered positive.
Figure 1.
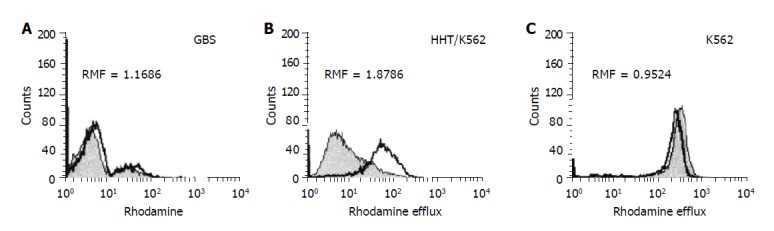
FACS analysis of Rh123 accumulation as a function of Pgp activity (A-C): (A) GBS were stained with a fluorescent substrate Rh123 in the presence (overlaid histogram) or absence (filled histogram) of the MDR reversing agent CyA. Pgp activity is expressed as RMF, a ratio of mean fluorescences obtained from the gated viable cell population. RMF ≥1 considered positive; (B) resistant (Pgp-positive/active) and (C) sensitive (Pgp-negative/inactive) control cell lines.
Variation due to test conditions Upon endoscopy, GBS was transported at a constant temperature of 4 °C and analyzed within 1 h. Gastric cells were mechanically dissociated by use of small scissors or by enzymatic digestion (trypsin), whereby the viability and gain were controlled by trypan blue exclusion. The cells obtained by mechanical dissociation (mincing with scissors) were more numerous and showed higher viability than those obtained by trypsin enzymatic dispersion. In order to assess the impact of storage on test reproducibility, 12 randomly chosen GBS were divided into two groups. One group was analyzed on the day of sampling, and the other was stored overnight at room temperature in 10 mL of cold buffer. The minimal concentration of GBS was 0.5×105 cells/mL.
Statistical analysis
We used STATISTICA software for the analysis of variance, multiple regression analysis and ROC analysis in the interpretation of results. The level of significance was set at P<0.001. The mean value of the scores obtained on antrum biopsy (anterior and posterior wall/2) was used for statistical analysis of DIEn and DHP.
RESULTS
Variation due to test conditions
The activity of Pgp was measured in GBS of 53 patients with H pylori infection and 12 dyspeptic patients without H pylori infection (control group). The two patient groups were matched according to mean age (47.39 vs 48.33 years) and sex (M/F = 5/7 vs 24/29).
In order to assess the impact of storage on test reproducibility, as shown in Table 1, the mean RMF was 1.427±1.034 and 2.521±2.067 on d 1 and 2, respectively. RMF was elevated in most of the samples (10/12; t test for dependent samples: t = 1.932; γ = 11; P<0.0795; SIGN test: z = 1.443; P<0.148). In the samples with the addition of modulator, fluorescence varied on d 2 and it seemed that there was no overexpression of Pgp function, although the cells were viable. As storage results in changed drug transport functions, for reasons of cellular metabolic and membrane integrity it is better to use fresh cells, within a few hours of sampling.
Table 1.
Effect of sample storage.
| H pylori positivepatients |
D1 |
D2 |
||||
| Rh123 MF | Rh123+CsA MF | RMF | Rh123 MF | Rh123+CsA MF | RMF | |
| 1 | 73.14 | 124.58 | 1.703 | 25.00 | 44.76 | 1.790 |
| 2 | 75.25 | 17.86 | 0.237 | 27.83 | 4.28 | 0.157 |
| 3 | 5.47 | 20.85 | 3.404 | 17.25 | 41.72 | 2.419 |
| 4 | 83.40 | 113.79 | 1.364 | 43.03 | 61.20 | 1.423 |
| 5 | 83.40 | 113.79 | 1.364 | 90.44 | 172.18 | 1.920 |
| 6 | 141.21 | 68.47 | 0.485 | 72.91 | 80.63 | 1.106 |
| 7 | 72.31 | 223.19 | 3.087 | 46.72 | 223.44 | 4.783 |
| 8 | 262.30 | 220.78 | 0.842 | 308.4 | 326.90 | 1.06 |
| 9 | 135.53 | 93.90 | 0.693 | 36.71 | 255.95 | 6.972 |
| 10 | 170.21 | 225.35 | 1.324 | 220.95 | 223.71 | 1.012 |
| 11 | 115.20 | 165.20 | 1.434 | 37.88 | 94.26 | 2.288 |
| 12 | 11.30 | 25.10 | 2.221 | 25.76 | 134.78 | 5.332 |
| RMF (mean±SD) | 1.427±1.034 | 2.522±2.067 | ||||
D 1 = fresh cells, several hours of sampling; d 2 = cells stored overnight at room temperature; Rh123 = rhodamine; CyA = cyclosporine A; MF = mean fluorescence; RMF = ratio of mean fluorescence.
Measurement of Pgp activity in patients with H pylori infection Pgp activity was determined in 53 patients with H pylori infection and 12 dyspeptic patients without H pylori infection (control group). The intensity of rhodamine dye extrusion by GBS with H pylori infection was higher as compared with H pylori negative control GBS (0.8±0.30 vs 1.38±0.66; γ = 1; F = 8.6, P<0.0046). H pylori infection stimulated Pgp activity. In patients with H pylori infection, RMF values were statistically increased significantly (P<0.005) as compared with control patients (Figure 2). Based on ROC analysis, RMF ≥1 was considered positive.
Figure 2.
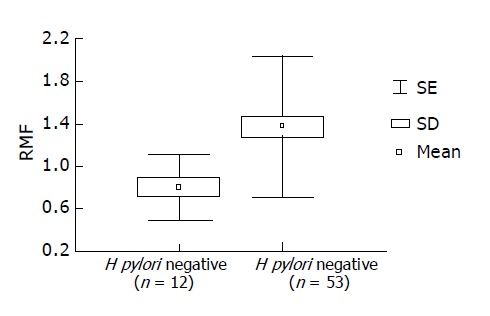
Enhanced Pgp activity in patients with H pylori infection. RMF values of 53 H pylori positive patients and 12 H pylori negative (control) patients are presented (x = mean; SD = standard deviation; SE = standard error).
Therapy stimulating Pgp activity Pre- and post-therapeutic Pgp activity was assessed in 10 randomly chosen H pylori positive patients irrespective of therapeutic success. After 7-wk treatment, stronger GBS extrusion of rhodamine dye was recorded in 6/10 patients, with a statistically significant increase in RMF values (Figure 3). Wilcoxon pair test yielded a significant difference between the pre- and post-therapeutic values (t = 9.0; z = 1.885; P<0.05).
Figure 3.
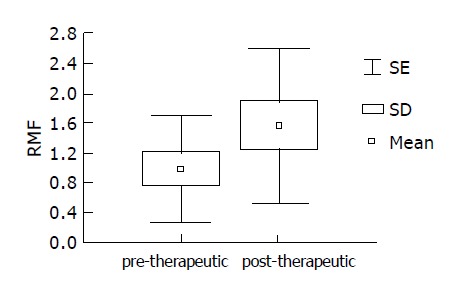
Therapeutic effect on Pgp activity in 10 randomly selected H pylori positive patients: significantly increased post-therapeutic RMF values.
Neither is repeat therapy efficacious in enhanced Pgp activity As shown in Table 2, the course of therapy was not affected by either age or sex. The patients requiring multiple courses at H pylori eradication (group B, n = 33) initially had a more severe infection (higher H pylori concentration and intraepithelial neutrophil infiltration), however, neither these variables were found crucial for course of treatment. Therapeutic success was influenced by Pgp activity: the patients with failure of H pylori eradication had a statistically higher (P<0.00001) pretherapeutic Pgp activity (1.647±0.65) than either patients with efficient H pylori eradication (0.889±0.28) or control group (0.787±0.29).
Table 2.
Role of pretherapeutic determination of Pgp activity.
| Variables |
Therapy cycle |
B/A and controls |
|||
|
H pylori positive |
H pylorinegative |
||||
| 1 = A (n = 20) | 2 or more = B (n = 33) | NO (n = 12) | Pa | Beta Pb | |
| Age (yr) | 47.5±8.8 | 47.3±13.7 | 48.3±16.5 | 0.973 | 0.152 |
| Sex (M/ F) | 9/11 | 15/18 | 5/7 | 0.975 | 0.153 |
| DHP | 2.75±0.91 | 2.90±0.97 | 1.0±0.0 | 0.00001 | 0.00065 |
| DIEn | 2.65±0.81 | 3.06±0.86 | 1.0±0.0 | 0.00001 | 0.45 |
| RMF (mean±SD) | 0.889±0.28 | 1.647±0.65 | 0.787±0.29 | 0.00001 | 0.000005 |
H pylori positive patients: A = one therapy cycle, B = two or more therapy cycles; NO = no therapy, M = male; F = female; DHP = H pylori density; DIEn = density of intraepithelial neutrophil infiltration; RMF = ratio of mean fluorescences, Pa for analysis of variance, Pb for multiple regression analysis.
Multiple regression analysis yielded a significantly highest beta coefficient for RMF and DIEn (P<0.001). ROC analysis demonstrated higher sensitivity, specificity and accuracy for RMF (90.90%, 71.87% and 81.54%, respectively, for borderline RMF value of 1.0) than for DHP (48%, 71% and 60%, respectively, for borderline DHP of score 2), and DIEn (66%, 71% and 69.23% respectively, for borderline DIEn of score 2).
Combined therapy has no effect in case of enhanced Pgp activity
Although the number of patients included in the study was too small for statistical analysis, the antibiotics used appeared to differ in stimulating Pgp activity, i.e. the first successful course with amoxicillin in combination with metronidazole or clarithromycin (RMF±SD for PAM 0.83±0.3 and for PAC 0.95±0.25) was less dependent on Pgp activity than the repeat combination with azithromycin (RMF±SD 1.706±0.62; n = 18). The patients with multiple attempts at H pylori eradication had higher pretherapeutic RMF values, however, there was no significant difference in the choice of antibiotics (between-group analysis of variance: f = 5.031; P<0.000898) (Figure 4).
Figure 4.
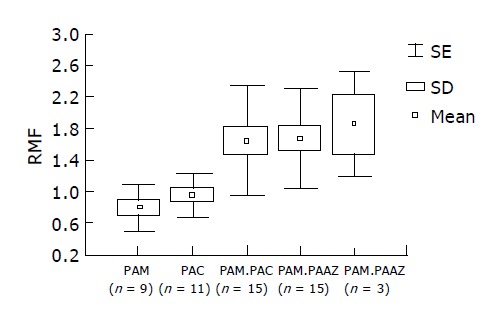
RMF values with five therapeutic protocols: combined therapy had no effect with enhanced Pgp activity. First course: PAM (pantoprazole +amoxicillin+metronidazole), PAC (pantoprazole+amoxicillin+clarithromycin); second course: PAM+PAC, PAM+PAAZ (pantoprazole+amoxicillin+azithromycin), PAC+PAAZ.
Factors influencing therapeutic efficacy Upon therapy completion, the effects of all parameters on the efficacy of H pylori eradication were assessed. Comparison of pretherapeutic RMF values between the patients with single H pylori eradication and those requiring two or more attempts at H pylori eradication showed no difference (P<0.13418). There was no significant difference in pretherapeutic Pgp activity between the patients with ultimately successful and unsuccessful H pylori eradication (1.26±0.715 vs 1.54±0.66). As there was no such difference in other clinical parameters either (age, sex, DIEn and DHP, P<0.189), it seems that, although influencing the duration and course of treatment, Pgp cannot be used to predict the ultimate treatment outcome (Table 3).
Table 3.
Impact of initial values on H pylori eradication efficacy.
| Variable |
Final eradication of H pylori |
P | |
| Successful (n = 31) | Unsuccessful (n = 22) | ||
| Age (yr) | 46.54±12.51 | 48.54±11.44 | 0.556 |
| Sex (M/F) | 15/16 | 9/13 | 0.598 |
| DHP | 2.90±0.97 | 2.77±0.92 | 0.626 |
| DIEn | 2.77±0.846 | 3.09±0.86 | 0.189 |
| RMF (mean±SD) | 1.26±0715 | 1.54±0.66 | 0.13418 |
M = male; F = female; DHP = H pylori density according to Sydney criteria; DIEn = density of intraepithelial neutrophil infiltration according to Sydney criteria; RMF = ratio of means.
DISCUSSION
Although H pylori infection is a well-characterized disease, many questions about the mechanisms of drug failures to cure the disease remain to be answered. The increased prevalence of antibiotic-resistant H pylori strains has serious implications, apart from patient compliance, as antimicrobial resistance is the most important factor determining the outcome of antibiotic therapy. The present article speculates about the potential mechanism limiting the gastrointestinal availability of antibiotics by Pgp mediated decrease in drug accumulation. Pgp is expressed on a wide variety of normal cells, especially in secretory tissues including the liver, intestinal tract epithelia from jejunum and colon, adrenal cortex, kidney, certain capillary endothelia, peripheral blood lymphocytes and hemopoietic precursor cells[28]. In gastrointestinal tract, Pgp acts as an ATP-consuming efflux pump extruding the natural toxins across the epithelial surface to the intestine, hepatocytes in biliary canaliculi and small ductules of the pancreas[29]. Direct evidence that Pgp inhibits the gastrointestinal absorption of orally administered drugs comes from several sources[30-33]. To the best of our knowledge, this is the first report analyzing Pgp activity in H pylori infection. We evaluated Pgp function in gastric cells from patients with H pylori infection by means of intracellular accumulation and efflux of a fluorescent dye. In the GBS loaded with Rh123 dye an increased efflux was observed in 33 H pylori positive patients who failed to respond to treatment. The patients with enhanced Pgp activity (RMF>1.5) failed to respond to either first or second therapy with the same or another antibiotic. As Pgp activity is low (RMF<1) in patients who respond favorably to the first course of treatment, it is of most importance to assess Pgp activity before therapy introduction and to monitor it before repeat antibiotic cycles. Pgp activity is generally low at the time of diagnosis, i.e. before the patient receives any treatment. These findings indicate that an increased Pgp activity may contribute to the drug-induced effect on gastric cells long after therapy completion. Although H pylori infection per se stimulates Pgp pump activity, this activity seems to additionally rise during the course of disease, because RMF index is lower at the disease onset. And the last but not the least, the lower RMF values recorded in patients with remission support the hypothesis that inappropriate therapeutic combinations lead to eradication failure. Therefore, Pgp activity testing might be used as a sign of therapeutic failure and need of multiple therapeutic protocols in a particular patient. Although Rh123 efflux could not predict final outcome of the attempts at H pylori eradication, it did show that elevated RMF values had greater impact on H pylori eradication failure with the use of AZ than with other antibiotics. Other parameters of H pylori infection (DHP, DIEn) did not prove useful in predicting either the need of an increased number of therapeutic protocols or the final treatment outcome.
Footnotes
Supported by Sveti Duh General Hospital, Zagreb, Croatia, approval, No. UR/P-10/1998
Science Editor Ma JY and Li WZ Language Editor Elsevier HK
References
- 1.Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection--the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 3.Georgopoulos SD, Ladas SD, Karatapanis S, Mentis A, Spiliadi C, Artikis V, Raptis SA. Factors that may affect treatment outcome of triple Helicobacter pylori eradication therapy with omeprazole, amoxicillin, and clarithromycin. Dig Dis Sci. 2000;45:63–67. doi: 10.1023/a:1005405209503. [DOI] [PubMed] [Google Scholar]
- 4.Megraud F. Resistance of Helicobacter pylori. In: Scarpignato C, Bianchi Porro G, Volume eds. Clinical pharmacology and therapy of Helicobacter pylori infection. Prog Basic Clin Pharmacol. Basel: Karger. 1999;11:329–352. [Google Scholar]
- 5.van der Hulst RWM, Tytgat GNJ. Treatment of Helicobacter pylori eradication failure. In: Scarpignato C, Bianchi Porro G, Volume eds, editors. Clinical pharmacology and therapy of Helicobacter pylori infection. Prog Basic Clin Pharmacol. Vol. 11. Basel: Karger; 1999. pp. 317–328. [Google Scholar]
- 6.van Doorn LJ, Schneeberger PM, Nouhan N, Plaisier AP, Quint WG, de Boer WA. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut. 2000;46:321–326. doi: 10.1136/gut.46.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin S. Clinical pharmacology of antimicrobials. In: Scarpignato C, Bianchi Porro G, Volume eds. Clinical pharmacology and therapy of Helicobacter pylori infection. Prog Basic Clinical Pharmacol. Basel: Karger. 1999;11:76–95. [Google Scholar]
- 8.Scarpignato C, Pelosini I. Antisecretory drugs for eradication of Helicobacter pylori: antibacterial activity and synergism with antimicrobial agents. In: Scarpignato C, Bianchi Porro G, Volume eds. Clinical pharmacology and therapy of Helicobacter pylori infection. Prog Basic Clin Pharmacol. Basel: Karger. 1999;11:136–178. [Google Scholar]
- 9.Peitz U, Menegatti M, Vaira D, Malfertheiner P. The European meeting on Helicobacter pylori: therapeutic news from Lisbon. Gut. 1998;43 Suppl 1:S66–S69. doi: 10.1136/gut.43.2008.s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba N, Hunt RH. Drug therapy of Helicobacter pylori infection: a meta-analysis. In: Scarpignato C, Bianchi Porro G, Volume eds. Clinical pharmacology and therapy of Helicobacter pylori infection. Prog Basic Clinical Pharmacol. Basel: Karger. 1999;11:227–268. [Google Scholar]
- 11.Pilotto A, Leandro G, Franceschi M, Rassu M, Bozzola L, Furlan F, Di Mario F, Valerio G. The effect of antibiotic resistance on the outcome of three 1-week triple therapies against Helicobacter pylori. Aliment Pharmacol Ther. 1999;13:667–673. doi: 10.1046/j.1365-2036.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- 12.Pilotto A, O’Morain C. Treatment of Helicobacter pylori infection. In: Malfetrheiner P, Megraud F, Michetti P, Price A, eds. The year of Helicobacter pylori 2000. Curr Opin Gastroenterol. 2000;16(Suppl 1):S44–S51. [Google Scholar]
- 13.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 14.van Veen HW, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen AJ, Konings WN. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci USA. 1996;93:10668–10672. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Veen HW, Konings WN. The ABC family of multidrug transporters in microorganisms. Biochim Biophys Acta. 1998;1365:31–36. doi: 10.1016/s0005-2728(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 16.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 17.McMurry L, Petrucci RE, Levy SB. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci USA. 1980;77:3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole K. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob Agents Chemother. 2000;44:2233–2241. doi: 10.1128/aac.44.9.2233-2241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putman M, van Veen HW, Konings WN. Molecular properties of bacterial multidrug transporters. Microbiol Mol Biol Rev. 2000;64:672–693. doi: 10.1128/mmbr.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, Iglewski BH, Storey DG. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2001;45:1761–1770. doi: 10.1128/AAC.45.6.1761-1770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cederbrant G, Kahlmeter G, Ljungh A. The E test for antimicrobial susceptibility testing of Helicobacter pylori. J Antimicrob Chemother. 1993;31:65–71. doi: 10.1093/jac/31.1.65. [DOI] [PubMed] [Google Scholar]
- 22.Megraud F, Henzel S, Glupczynski Y, Antibiotic susceptibility and resistance in Helicobacter pylori: physiology and genetics. ASM Press. 2001:511–30. [Google Scholar]
- 23.Chaves S, Gadanho M, Tenreiro R, Cabrita J. Assessment of metronidazole susceptibility in Helicobacter pylori: statistical validation and error rate analysis of breakpoints determined by the disk diffusion test. J Clin Microbiol. 1999;37:1628–1631. doi: 10.1128/jcm.37.5.1628-1631.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Katicic M, Presecki V, Marusic M. Eradication of Helicobacter pylori infection with two triple regimens of 7, 10 and 14 d duration (abstract) Gut. 1997;41(Suppl 1):A100. [Google Scholar]
- 26.Marie JP, Huet S, Faussat AM, Perrot JY, Chevillard S, Barbu V, Bayle C, Boutonnat J, Calvo F, Campos-Guyotat L, et al. Multicentric evaluation of the MDR phenotype in leukemia. French Network of the Drug Resistance Intergroup, and Drug Resistance Network of Assistance Publique-Hôpitaux de Paris. Leukemia. 1997;11:1086–1094. doi: 10.1038/sj.leu.2400656. [DOI] [PubMed] [Google Scholar]
- 27.Broxterman HJ, Lankelma J, Pinedo HM, Eekman CA, Währer DC, Ossenkoppele GJ, Schuurhuis GJ. Theoretical and practical considerations for the measurement of P-glycoprotein function in acute myeloid leukemia. Leukemia. 1997;11:1110–1118. doi: 10.1038/sj.leu.2400685. [DOI] [PubMed] [Google Scholar]
- 28.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol. 1997;8:161–170. doi: 10.1006/scbi.1997.0068. [DOI] [PubMed] [Google Scholar]
- 30.Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27:161–170. doi: 10.1016/s0169-409x(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 31.Leu BL, Huang JD. Inhibition of intestinal P-glycoprotein and effects on etoposide absorption. Cancer Chemother Pharmacol. 1995;35:432–436. doi: 10.1007/s002800050258. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res. 1996;13:963–977. doi: 10.1023/a:1016086003070. [DOI] [PubMed] [Google Scholar]
- 33.Pelkonen O, Boobis AR, Gundert-Remy U. In vitro prediction of gastrointestinal absorption and bioavailability: an experts' meeting report. Eur J Clin Pharmacol. 2001;57:621–629. doi: 10.1007/s002280100369. [DOI] [PubMed] [Google Scholar]


