Abstract
The effects of phagocytosis on plasma membrane microviscosity were studied by fluorescence depolarization techniques. It was shown that lipophilic probes are accumulated in intracellular vesicles to a significant degree in fibroblasts and neutrophils. Microviscosity was thus determined from the behavior of probes in isolated membranes. Phagocytosis of oil emulsions or polystyrene beads by rabbit polymorphonuclear leukocytes induces a marked decrease in plasma membrane microviscosity that parallels the extent of phagocytosis. Liposomes made from extracts of membrane lipid show qualitatively the same changes, indicating that the alteration of microviscosity results at least in part from changes in lipid composition. The decrease in microviscosity is abolished when colchicine is present during phagocytosis. Addition of colchicine to membranes previously isolated from control or phagocytic cells has no effect on their microviscosity. The results suggest that phagocytosis is accompanied by a microtubule-dependent reorganization of membrane lipids.
Full text
PDF

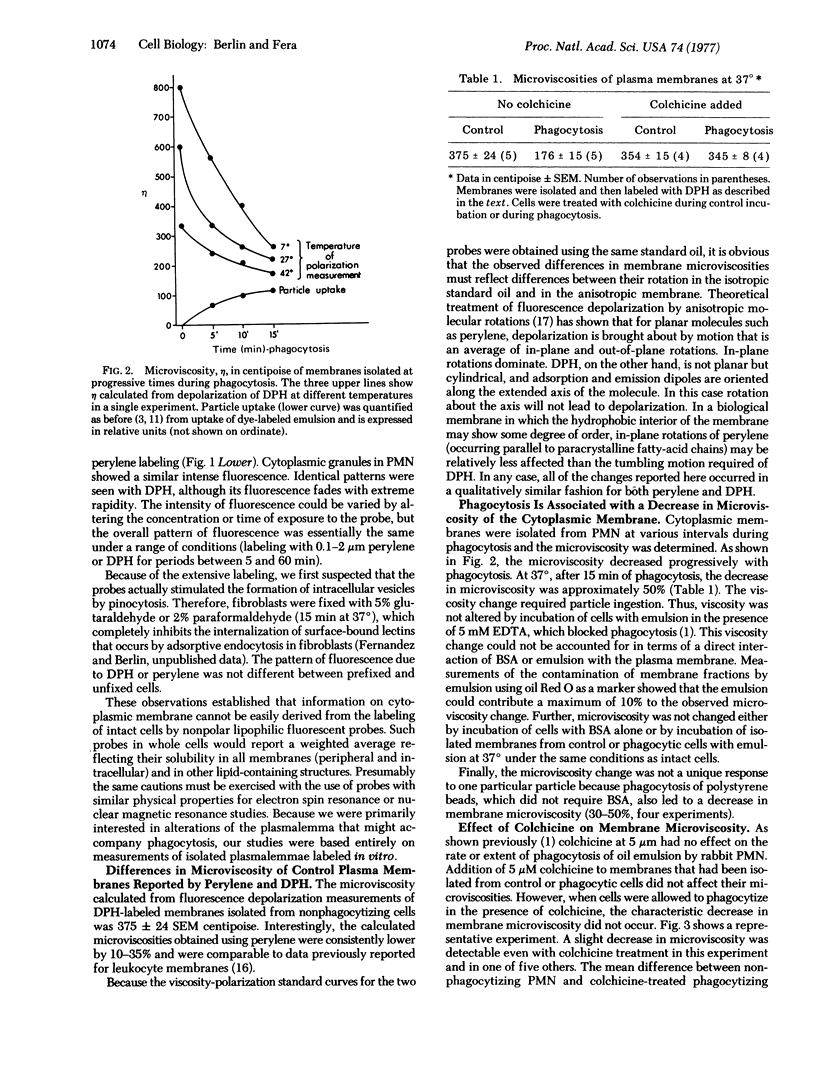
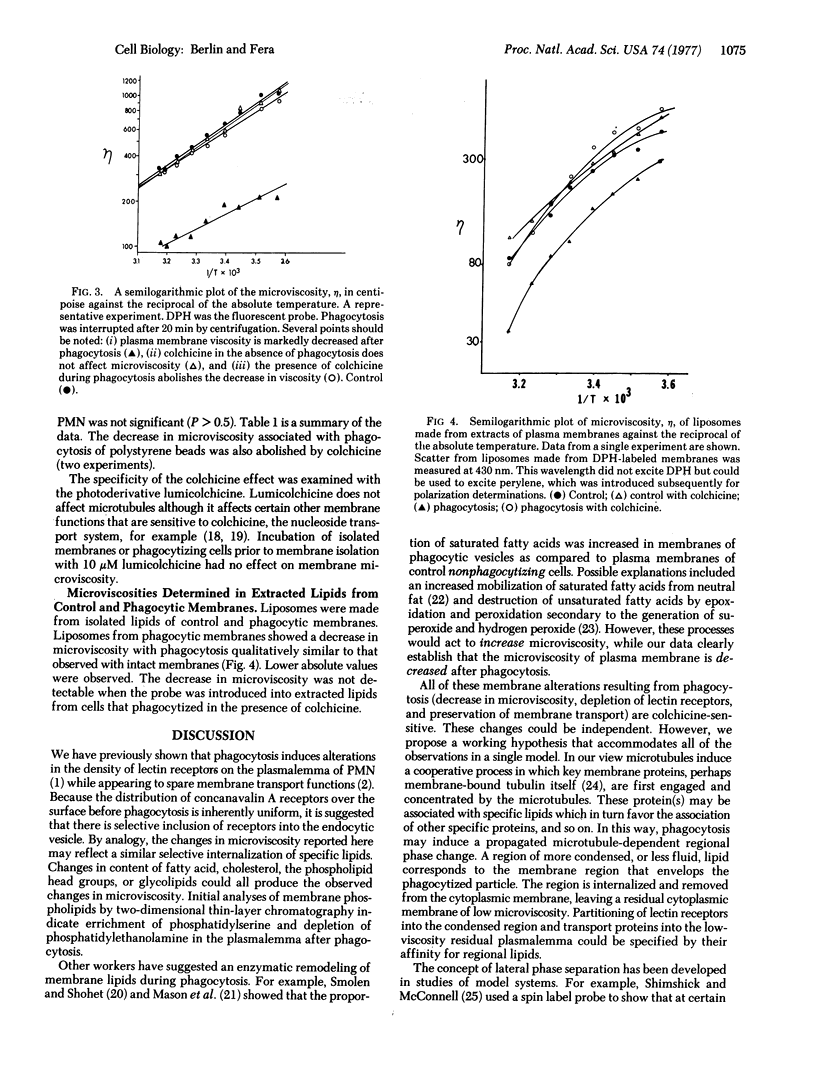

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett R. E., Scott R. E., Furcht L. T., Kersey J. H. Evidence that mitogenic lectins induce changes in lymphocyte membrane fluidity. Nature. 1974 May 31;249(456):465–466. doi: 10.1038/249465a0. [DOI] [PubMed] [Google Scholar]
- Berlin R. D. Temperature dependence of nucleoside membrane transport of rabbit alveolar macrophages and polymorphonuclear leukocytes. J Biol Chem. 1973 Jul 10;248(13):4724–4730. [PubMed] [Google Scholar]
- Bhattacharyya B., Volff J. Membrane-bound tubulin in brain and thyroid tissue. J Biol Chem. 1975 Oct 10;250(19):7639–7646. [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R. Oxidative bactericidal mechanisms of polymorphonuclear leukocytes. J Infect Dis. 1975 Mar;131(3):295–303. doi: 10.1093/infdis/131.3.295. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. II. Properties and suitability as markers for the plasma membrane. J Biol Chem. 1974 Nov 25;249(22):7121–7129. [PubMed] [Google Scholar]
- Elsbach P., Farrow S. Cellular triglyceride as a source of fatty acid for lecithin synthesis during phagocytosis. Biochim Biophys Acta. 1969 Mar 4;176(2):438–441. doi: 10.1016/0005-2760(69)90208-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Feinstein M. B., Fernandez S. M., Sha'afi R. I. Fluidity of natural membranes and phosphatidylserine and ganglioside dispersions. Effect of local anesthetics, cholesterol and protein. Biochim Biophys Acta. 1975 Dec 16;413(3):354–370. doi: 10.1016/0005-2736(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Fuchs P., Parola A., Robbins P. W., Blout E. R. Fluorescence polarization and viscosities of membrane lipids of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3351–3354. doi: 10.1073/pnas.72.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Soberman R., Goldstein I., Weissmann G. Concanavalin A induces microtubule assembly and specific granule discharge in human polymorphonuclear leukocytes. J Cell Biol. 1976 Mar;68(3):781–787. doi: 10.1083/jcb.68.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Pagano R. E. Interaction of phospholipid vesicles with cultured mammalial cells. I. Characteristics of uptake. J Cell Biol. 1975 Oct;67(1):38–48. doi: 10.1083/jcb.67.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER H. K., WOOD W. B., Jr Studies on the pathogenesis of fever. X. The effect of certain enzyme inhibitors on the production and activity of leucocvtic pvrogen. J Exp Med. 1962 Jan 1;115:37–47. doi: 10.1084/jem.115.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Lateral phase separations in Escherichia coli membranes. Biochim Biophys Acta. 1974 Apr 29;345(2):220–230. doi: 10.1016/0005-2736(74)90260-0. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Stossel T. P., Vaughan M. Lipids of alveolar macrophages, polymorphonuclear leukocytes, and their phagocytic vesicles. J Clin Invest. 1972 Sep;51(9):2399–2407. doi: 10.1172/JCI107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Albertini D. F., Berlin R. D. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976 Dec;71(3):921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Ukena T. E., Berlin R. D. Effects of phagocytosis and colchicine on the distribution of lectin-binding sites on cell surfaces. Proc Natl Acad Sci U S A. 1974 Feb;71(2):394–398. doi: 10.1073/pnas.71.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E., Vail W. J. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim Biophys Acta. 1974 May 30;352(1):10–28. doi: 10.1016/0005-2736(74)90175-8. [DOI] [PubMed] [Google Scholar]
- Poste G., Papahadjopoulos D. Lipid vesicles as carriers for introducing materials into cultured cells: influence of vesicle lipid composition on mechanism(s) of vesicle incorporation into cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1603–1607. doi: 10.1073/pnas.73.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. PARTICLE UPTAKE BY POLYMORPHONUCLEAR LEUCOCYTES AND EHRLICH ASCITES-CARCINOMA CELLS. Biochem J. 1963 Oct;89:150–156. doi: 10.1042/bj0890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Cramer R., Rossi F. Use of 1-anilino-8-naphtalene sulfonate to study structural transitions in cell membrane of PMN leucocytes. Biochem Biophys Res Commun. 1970 Nov 9;41(3):582–588. doi: 10.1016/0006-291x(70)90052-5. [DOI] [PubMed] [Google Scholar]
- Rudy B., Gitler C. Microviscosity of the cell membrane. Biochim Biophys Acta. 1972 Oct 23;288(1):231–236. doi: 10.1016/0005-2736(72)90242-8. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Shinitzky M., Dianoux A. C., Gitler C., Weber G. Microviscosity and order in the hydrocarbon region of micelles and membranes determined with fluorescent probes. I. Synthetic micelles. Biochemistry. 1971 May 25;10(11):2106–2113. doi: 10.1021/bi00787a023. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Shohet S. B. Remodeling of granulocyte membrane fatty acids during phagocytosis. J Clin Invest. 1974 Mar;53(3):726–734. doi: 10.1172/JCI107611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Effect of phagocytosis on membrane transport of nonelectrolytes. J Exp Med. 1971 Oct 1;134(4):1016–1035. doi: 10.1084/jem.134.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena T. E., Berlin R. D. Effect of colchicine and vinblastine on the topographical separation of membrane functions. J Exp Med. 1972 Jul 1;136(1):1–7. doi: 10.1084/jem.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]