Abstract
AIM: To analyze the expression levels of soluble form of CD95, CD95 ligand (sCD95 and sCD95L, respectively) in plasma and CD95 expression on CD3+ cells in liver-transplanted recipients with acute rejection (AR).
METHODS: Peripheral blood mononuclear cells (PBMCs) were isolated from 30 clinically liver transplanted recipients. CD95 expression on CD3+ cells was quantitatively measured by two-color fluorescence activated cell sorter (FACS) analysis. Lymphocyte surface phenotypes of CD4, CD8, CD16 and CD56 were determined by flow cytometry. Plasma levels of sCD95 and sCD95L were detected by Enzyme Linked-Immuno-Sorbent Assay (ELISA). The results were compared with that from normal healthy volunteers (n = 15 individuals).
RESULTS: FACS analysis showed that CD95 expression on CD3+ T cells was significantly increased in liver transplanted recipients with AR compared to that in stable recipients without rejection and infection or healthy individuals who did not undergo transplantation (18676.93±11588.34/molecule, 6848.20±1712.96/molecule, 6418.01±2001.95/molecule, respectively, P<0.01). Whereas no significant difference was seen between liver-transplanted stable recipients and healthy individuals. Furthermore, no significant differences were detected between each group with CD4/CD8 ratio or the percentage of CD16+56+ cells. Plasma levels of sCD95 were significantly higher in transplanted recipients with AR compared to that in stable recipients or healthy individuals (391.88±196.00, 201.37±30.30, 148.83±58.25 pg/mL, respectively, P<0.01). In contrast, the plasma levels of sCD95L in liver- transplanted recipients were not significantly different from that in healthy individuals.
CONCLUSION: The present results indicate that the increased CD95 expression on CD3+ cells and the increased levels of sCD95 in plasma may modify the immunological situation of the recipients after transplantation or represent the ongoing graft rejection.
Keywords: Liver transplantation, Acute rejection, CD95
INTRODUCTION
Numerous studies have proposed several mechanisms of graft rejection in liver transplantation such as the manipulation of intragraft cytokines, apoptosis of graft infiltrating lymphocyte and so on. Cytokines play an important role in regulating immunological responses of the host against transplanted organs. They control the activation and differentiation of immune effector cells and mediate cytotoxic activity of the effector cells. It has been suggested that Type I CD4+ and CD8+ T cells (Th1, Tc1) cytokines (interleukin-2, interferon-γ and tumor necrosis factor (TNF)-α) might promote cellular rejection[1-3], whereas type II CD4+ and CD8+ T cells (Th2, Tc2) cytokines (interleukin-4 and interleukin-10) might suppress graft rejection[4].
T cells stimulated via the T-cell receptors (TCRs) not only proliferate, but also undergo subsequent apoptosis by activation-induced cell death (AICD)[5,6]. Much evidence indicates the implication of AICD in the immune responses against alloantigens. Recent studies have been reporting that some pathways involved AICD of T cells[7,8]. CD95/CD95 ligand (CD95L) pathway has been shown to be a major AICD mediator of T cells. CD95 (Fas or APO-1) and its ligand are cell surface proteins. CD95 is a 48-KD type I transmembrane protein member belonging to the TNF receptor family, and CD95L is a 40-KD type II integral membrane protein belonging to the TNF family[9]. The interaction between the CD95 and CD95L is recognized as a major pathway for the induction of apoptosis in cells and tissues[10]. It has been suggested that the CD95 and CD95L play an important role in the regulation of immune responses to foreign antigens and in the induction of peripheral tolerance[11]. sCD95 has recently been detected in the serum of the patients with liver diseases, including injury, hepatitis, cirrhosis, hepatocellular carcinoma (HCC) and in the systemic lupus erythematosus patients[12,13]. It has been shown that human CD95L was secreted from activated T cells[14] and the sCD95L may act as a cytotoxic cytokine, although Tanaka and colleagues recently suggested its inhibitory function[15]. CD95/CD95L pathway is regulated by a number of implicating factors. Till now, the role of CD95 and CD95 ligand system in graft rejection is not fully understood and changes of their expression during liver allograft rejection have not been elucidated.
Given the above considerations, we aimed to examine CD95 expression by CD3+ T cells and the plasma levels of sCD95 and sCD95L in human recipients after liver transplantation, and estimated their relation to the liver rejection. The significance of these results on liver transplantation will be discussed below.
MATERIALS AND METHODS
Patient
Thirty liver -transplanted recipients (24 men, 6 women) who were treated at Organ Transplantation Center of Tianjin First Central Hospital were divided into post-transplanted AR (n = 15) and post-transplanted stable (n = 15) groups. The patients had been transplanted within 3 mo of immunosuppressed treatment with FK506, zenapax, MMF, and steroids. AR was diagnosed by means of clinical, laboratory, and histologic evidence. Methylprednisolone was used for treatment. Blood samples were collected from AR group and stable group after liver- transplantation. The control group consisted of 15 healthy individuals. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque density gradient centrifugation from ethylenediaminetetraacetic acid (EDTA) blood 5 mL. Plasma was collected from healthy donors and patients by centrifugation of heparinized peripheral blood (PB) at 3000 r/min for 10 min. Plasma samples were divided into aliquots and stored at -70 °C until measured.
Methods
Plasma cytokines The concentration of sCD95 in plasma was determined by a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (ELISA) (DIACLONE France). Briefly, a monoclonal antibody (mAb) specific for sCD95 has been coated onto the wells of the microliter strips provided. Plasma and standards of known sCD95 concentrations are pipetted into these wells. During the first incubation, the antigen and a biotinylated mAb specific for sCD95 are simultaneously incubated. After five washes with 0.05% Tween 20-phosphated buffered saline (PBS), pH7.4, the enzyme (streptavidin-peroxydase) is added. After incubation and washing, to remove all unbound enzyme, a substrate solution, which acts with the bound enzyme, is added to induce a colored product. The intensity of this colored product is directly proportional to the concentration of sCD95 present in the plasma. The same ELISA system for sCD95L was used for the in vitro quantitative determination of sCD95L in plasma.
Determination of lymphocyte subpopulations Staining with mAb was performed in 100 μL aliquots of heparinized PB, followed by lysis of red blood cells and fixation with 1% paraformaldehyde (PAF). Phycoerythrin (PE) conjugated mouse anti-human CD4, CD8, CD16, fluorescein isothiocyanate (FITC) conjugated mouse anti-human CD56 and isotype-matched control mAbs (All from Becton Dickinson) were used. 10000 events were acquired using fluorescence activated cell sorter (FACS) Calibur and analysis was performed using cellquest software (Becton Dickinson).
Quantitative measurement of cell surface expression of CD95 (Fas/Apo-1) expression on CD3+ lymphocytes by dual-color flow cytometry Fifty microliter of cell suspension was added to each of 2-5 mL polystyrene snap cap tubes. To the first tube (T1), 25 μL negative isotypic control (BIOCYTEX, France) was added. To the second tube (T2), 25 μL FITC conjugated mouse anti-human CD95 (Fas/Apo-1) mAb (BIOCYTEX) was added. To the third tube (T3), 50 μL of QuantiBRITE beads (BDB) suspension (BIOCYTEX), which were coated with increasing and accurately known quantities of mouse immunoglobulins G was added. Samples were incubated for 10 min at room temperature. Subsequently, in each of the tube, 10 μL of PE conjugated mouse anti-human CD3 mAb (Becton Dickinson) was added. Samples were incubated at room temperature for 10 min, followed by washing in PBS with 2% fetal calf serum and fixation in 1% PAF and then analyzed by flow cytometry. The standard curve (Figure 1C) was made by plot the MFI (mean fluorescence index) calibration values obtained from T3 on the X-axis and their corresponding number of mAb molecules on the Y-axis (Figure 1B). Note the MFI values of T1 and T2 obtained on the corresponding histogram after gating CD3+ cells (Figure 1A). Interoplate the MFI values of T1 and T2, then read the corresponding number of mAbs directly off the curve. The number of specific sites was obtained by subtracting T1 value to T2 value.
Figure 1.
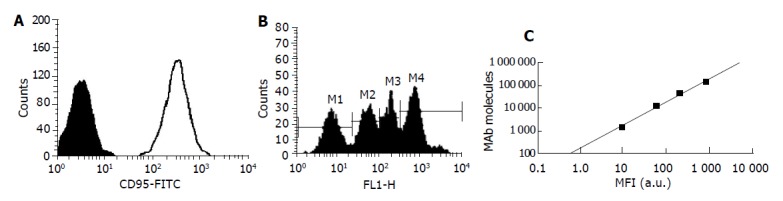
Quantitative measurement of cell surface expression of CD95 expression on CD3+ lymphocytes PBMCs were labeled with QuantiBRITE beads suspension (T3), CD95-FITC (T2) or isotype control IgG1-FITC (T1) and CD3-PE as described in materials and methods. A: Histogram showed the expression of CD95 on CD3+ cells. Solid histogram is isotype control. Bold line: CD95; B: Histogram showed the fluorescence intensity of the mouse immunoglobulins G in T3; C: The standard curve was made by plot the MFI calibration values obtained from Figure 1B on the X-axis and their corresponding number of mAb molecules on the Y-axis.
Statistical analysis
All data are presented as the mean±SD. Statistic analyses were performed with the t-tests. P-values of less than 0.05 were regarded as significant throughout the study.
RESULTS
We analyzed and compared freshly isolated PBMCs from liver-transplanted patients and healthy individuals as regards CD95 expression on CD3+ T cell by dual-color flow cytometry (Figure 1). We did not find any significant difference between post-transplanted stable recipients and healthy individuals. However, CD95 expression on CD3+ T cells was significantly increased in liver transplanted recipients with AR compared with that in stable recipients without rejection and infection or healthy individuals who did not undergo transplantation (P<0.01, Table 1).
Table 1.
Levels of CD95 expression on CD3+cells (/molecule) and levels of sCD95, sCD95L (pg/mL) in plasma from post-transplanted AR group, post-transplanted stable group and healthy group.
| Post-transplantedstable group (n = 15) | Post-transplantedAR group (n = 15) | Healthy group (n = 15) | |
| CD95 (/molecule) | 6 848.20±1 712.96b | 18 676.93±11 588.34 | 6 418.01±2 001.95d |
| sCD95 (pg/mL) | 201.37±30.30b | 391.88±196.00 | 148.83±58.25d |
| sCD95L (pg/mL) | 279.42±79.72 | 226.82±132.63 | 254.38±96.29 |
P<0.01 vs post-transplanted stable group;
P<0.01 vs health group.
We also investigated the sCD95 and sCD95L in plasma by ELISA. Plasma levels of sCD95 were significantly higher in transplanted recipients with AR than that in stable recipients or healthy individuals (P<0.01). In contrast, the levels of sCD95L in liver transplanted recipients were not significantly different from that expressed in healthy individuals (Table 1).
As concerns T lymphocyte subpopulations, no significant differences were seen among each group with CD4/CD8 ratio and the percentage of CD16+56+ cells (Table 2).
Table 2.
CD4/CD8 ratio, the percentage of CD16+56+ cells in post-transplanted AR group, post-transplanted stable group and healthy group.
| Post-transplanted stable group (n = 15) | Post-transplanted AR group (n = 15) | Healthy group (n = 15) | |
| CD4/CD8 | 1.5±0.5 | 1.8±0.4 | 1.5±0.2 |
| CD16+56+ (%) | 15.4±9.5 | 20.4±9.4 | 17.0±7.5 |
DISCUSSION
Acute rejection (AR) is a well-known complication of allograft transplantation. T lymphocytes appear to be absolutely required in this rejection process[16,17]. Fas (CD95) is expressed in resting peripheral blood T cells and the Fas antigen as well as the ligand (FasL, CD95L) are up-regulated following T cell activation. Increased expression of FasL may activate the cytotoxic pathway, leading to the graft damage by Fas system activation and result in apoptosis.
The studies of Rivero et al[18], showed that up-regulated expression of Fas antigen in liver tissue with liver rejection but not in liver tissue without rejection, suggesting the importance of the Fas system in the rejection of liver grafts. Our studies, which focused on the peripheral blood lymphocytes, showed that CD95 expression on CD3+ cells did not have any significant difference between liver- transplanted stable recipients without rejection and infection and healthy individuals who did not undergo transplantation. This result was similar to that of Renzo, who demonstrated that Fas on CD3+ T cell and FasL mRNA expression in PBMC have no significant difference between cardiac- transplanted subjects and normal controls[19]. However, when compared to the recipients with AR to the stable recipients or healthy individuals, we found that the CD95 expression on CD3+ cells was significantly increased. This increasing may have been caused by antigen stimulation. Stimulation through the CD3-TCR complex up-regulates CD95 expression and induces CD95L expression[20,21]. Through these cell surface molecules, activated T cells can commit suicide through formation of CD95-CD95L complexes[22,23]. Therefore, we suppose that because of the higher CD95 expression, CD3+ T lymphocyte in AR patients may undergo more apoptosis than that from stable recipients, so that the spontaneous tolerance to the allograft may develop. On the other hand, previous animal studies of cardiac, renal and liver transplantation demonstrated that FasL up-regulation in allografts in rejection condition[24,25]. Therefore, we cannot exclude that in AR patients, the CD95 higher expression T lymphocyte may infiltrate in the allograft and induce apoptosis through formation of CD95-CD95L complex, then accelerate the rejection process.
In the meantime, we determined the plasma levels of sCD95 in liver- transplantation recipients. The sCD95 results from the deletion of the transmembrane domain of CD95. The levels of sCD95 were significantly increased in liver transplanted recipients with AR compared to that in stable recipients or healthy individuals. It has been speculated that sCD95 may reflect the expression levels of Fas antigen on tissue and the severity of inflammatory activity[26-30]. Therefore, the sCD95 detected in the present samples might be shedding from injured organs that express CD95. The significant increase of sCD95 in AR recipients were strong associated with the serum levels of total bilirubin, AST and ALT (unpublished data), implying that the levels of sCD95 may reflect the graft damage, which could be useful when evaluating response to treatment. A previous study demonstrated the expression of CD95L mRNA in kidney-transplanted grafts at AR[31]. However, in this study, plasma levels of sCD95L in liver- transplanted recipients were not significantly different from that in healthy individuals, indicating that sCD95L might not contribute to AR. Concomitant immunosuppression, which is routinely administered in human transplantation cases, must also be a main cause for no increasing the FasL expression in rejection condition. Further, studies will be needed to elucidate the role of sCD95L in chronic rejection.
T lymphocytes can be separated into two sub-sets based on their expression of the CD4 and CD8 molecules on the cell surface. There are contradictory opinions on the relative contribution of CD4+ and CD8+ T cells to allograft rejection. Some animal models indicate that there is an absolute requirement for CD4+ T cells in allogeneic rejection, whereas in others CD4-depleted mice reject certain types of allografts[32]. Haskova et al used CD4- or CD8-deficient knockout mice to investigate the role of T cell subsets in allograft rejection. The results showed that CD4+ T cells play a critical role in the rejection of corneal allografts, whereas CD8+ T cells appear to be involved in the rejection of skin allografts[33]. In our study, the CD4/CD8 ratio had showed no significant difference among AR group, stable group and healthy group. This finding is not compatible with that of Sadeghi et al[34] who showed that T lymphocyte sub-populations were lower in rejecting than in non-rejecting patients after renal transplantation. This difference may result from different organ allograft. Further studies will be needed to better understand the role of CD4 and CD8 T lymphocyte in the allograft rejection.
Human natural killer (NK) cells are defined by their ability to lyse target cells without prior sensitization and without restriction by major histocompatibility (MHC) antigens. It has been shown that these cells play an important role in immune defenses, especially after hematopoietic transplantation[35]. In our study, we demonstrated that the percentage of CD16+CD56+ cells did not change in AR patients, implying that NK cells may not play an important role in AR in liver transplantation or the immunosuppressive therapy interfere with the generation of NK cells.
In conclusion, monitoring of CD95 on CD3+ T cells and in plasma may provide an important clue to a better understanding of the pathogenesis of liver graft rejection and would be a helpful tool to develop new therapeutic approaches for the prevention of AR by controlling the CD95/CD95L signaling.
ACKNOWLEDGEMENTS
We thank Dr. Wei Gao from Organ Transplantation Center of Tianjin First Central Hospital for their clinical guidance.
Footnotes
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Gupta RK, Jain M, Sharma RK. Serum & amp; urinary interleukin-2 levels as predictors in acute renal allograft rejection. Indian J Med Res. 2004;119:24–27. [PubMed] [Google Scholar]
- 2.Wang YL, Tang ZQ, Gao W, Jiang Y, Zhang XH, Peng L. Influence of Th1, Th2, and Th3 cytokines during the early phase after liver transplantation. Transplant Proc. 2003;35:3024–3025. doi: 10.1016/j.transproceed.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Bathgate AJ, Lee P, Hayes PC, Simpson KJ. Pretransplantation tumor necrosis factor-alpha production predicts acute rejection after liver transplantation. Liver Transpl. 2000;6:721–727. doi: 10.1053/jlts.2000.18472. [DOI] [PubMed] [Google Scholar]
- 4.Drannik GN, Lunova AG, Baran YY, Poroshina TV, Kalinina NA. Cytokine producing function of type II T-helpers in transplanted kidney patients. Transplant Proc. 2001;33:2352–2354. doi: 10.1016/s0041-1345(01)02019-x. [DOI] [PubMed] [Google Scholar]
- 5.Radvanyi LG, Mills GB, Miller RG. Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J Immunol. 1993;150:5704–5715. [PubMed] [Google Scholar]
- 6.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338–4345. [PubMed] [Google Scholar]
- 7.Lai JH, Ho LJ, Lu KC, Chang DM, Shaio MF, Han SH. Western and Chinese antirheumatic drug-induced T cell apoptotic DNA damage uses different caspase cascades and is independent of Fas/Fas ligand interaction. J Immunol. 2001;166:6914–6924. doi: 10.4049/jimmunol.166.11.6914. [DOI] [PubMed] [Google Scholar]
- 8.Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. 2001;166:4931–4942. doi: 10.4049/jimmunol.166.8.4931. [DOI] [PubMed] [Google Scholar]
- 9.Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173–184. doi: 10.1006/scbi.2000.0318. [DOI] [PubMed] [Google Scholar]
- 10.Boussiotis VA, Lee BJ, Freeman GJ, Gribben JG, Nadler LM. Induction of T cell clonal anergy results in resistance, whereas CD28-mediated costimulation primes for susceptibility to Fas- and Bax-mediated programmed cell death. J Immunol. 1997;159:3156–3167. [PubMed] [Google Scholar]
- 11.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 12.Sacco R, Leuci D, Tortorella C, Fiore G, Marinosci F, Schiraldi O, Antonaci S. Transforming growth factor beta1 and soluble Fas serum levels in hepatocellular carcinoma. Cytokine. 2000;12:811–814. doi: 10.1006/cyto.1999.0650. [DOI] [PubMed] [Google Scholar]
- 13.Bijl M, Horst G, Limburg PC, Kallenberg CG. Fas expression on peripheral blood lymphocytes in systemic lupus erythematosus (SLE): relation to lymphocyte activation and disease activity. Lupus. 2001;10:866–872. doi: 10.1191/096120301701548517. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, et al. Fas ligand in human serum. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 16.Krensky AM, Weiss A, Crabtree G, Davis MM, Parham P. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med. 1990;322:510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 17.Zavazava N, Kabelitz D. Alloreactivity and apoptosis in graft rejection and transplantation tolerance. J Leukoc Biol. 2000;68:167–174. [PubMed] [Google Scholar]
- 18.Rivero M, Crespo J, Mayorga M, Fábrega E, Casafont F, Pons-Romero F. Involvement of the Fas system in liver allograft rejection. Am J Gastroenterol. 2002;97:1501–1506. doi: 10.1111/j.1572-0241.2002.05797.x. [DOI] [PubMed] [Google Scholar]
- 19.Di Renzo M, Capecchi PL, Camurri A, Di Ciolla F, Maccherini M, Lisi G, Pompella G, Pasqui AL, Auteri A, Abbracchio MP, et al. Enhanced apoptosis of peripheral blood mononuclear cells in cardiac transplanted patients undergoing chronic immunosuppressive treatment. Transpl Immunol. 2002;10:269–275. doi: 10.1016/s0966-3274(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 20.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 22.Bonfoco E, Stuart PM, Brunner T, Lin T, Griffith TS, Gao Y, Nakajima H, Henkart PA, Ferguson TA, Green DR. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity. 1998;9:711–720. doi: 10.1016/s1074-7613(00)80668-8. [DOI] [PubMed] [Google Scholar]
- 23.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 24.Seino K, Kayagaki N, Bashuda H, Okumura K, Yagita H. Contribution of Fas ligand to cardiac allograft rejection. Int Immunol. 1996;8:1347–1354. doi: 10.1093/intimm/8.9.1347. [DOI] [PubMed] [Google Scholar]
- 25.Josien R, Müschen M, Gilbert E, Douillard P, Heslan JM, Soulillou JP, Cuturi MC. Fas ligand, tumor necrosis factor-alpha expression, and apoptosis during allograft rejection and tolerance. Transplantation. 1998;66:887–893. doi: 10.1097/00007890-199810150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Crespo J, Rivero M, Mayorga M, Fabrega E, Casafont F, Gomez-Fleitas M, Pons-Romero F. Involvement of the fas system in hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2000;6:562–569. doi: 10.1053/jlts.2000.9742. [DOI] [PubMed] [Google Scholar]
- 27.Ryo K, Kamogawa Y, Ikeda I, Yamauchi K, Yonehara S, Nagata S, Hayashi N. Significance of Fas antigen-mediated apoptosis in human fulminant hepatic failure. Am J Gastroenterol. 2000;95:2047–2055. doi: 10.1111/j.1572-0241.2000.02268.x. [DOI] [PubMed] [Google Scholar]
- 28.Iio S, Hayashi N, Mita E, Ueda K, Mochizuki K, Hiramatsu N, Kanto T, Sasaki Y, Kasahara A, Hori M. Serum levels of soluble Fas antigen in chronic hepatitis C patients. J Hepatol. 1998;29:517–523. doi: 10.1016/s0168-8278(98)80145-1. [DOI] [PubMed] [Google Scholar]
- 29.Hamzaoui K, Hamzaoui A, Zakraoui L, Chabbou A. Levels of soluble Fas/APO-1 in patients with Behçet's disease. Mediators Inflamm. 1998;7:111–114. doi: 10.1080/09629359891261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nozawa K, Kayagaki N, Tokano Y, Yagita H, Okumura K, Hasimoto H. Soluble Fas (APO-1, CD95) and soluble Fas ligand in rheumatic diseases. Arthritis Rheum. 1997;40:1126–1129. doi: 10.1002/art.1780400617. [DOI] [PubMed] [Google Scholar]
- 31.Sharma VK, Bologa RM, Li B, Xu GP, Lagman M, Hiscock W, Mouradian J, Wang J, Serur D, Rao VK, et al. Molecular executors of cell death--differential intrarenal expression of Fas ligand, Fas, granzyme B, and perforin during acute and/or chronic rejection of human renal allografts. Transplantation. 1996;62:1860–1866. doi: 10.1097/00007890-199612270-00031. [DOI] [PubMed] [Google Scholar]
- 32.Fischbein MP, Yun J, Laks H, Irie Y, Fishbein MC, Espejo M, Bonavida B, Ardehali A. CD8+ lymphocytes augment chronic rejection in a MHC class II mismatched model. Transplantation. 2001;71:1146–1153. doi: 10.1097/00007890-200104270-00023. [DOI] [PubMed] [Google Scholar]
- 33.Haskova Z, Usiu N, Pepose JS, Ferguson TA, Stuart PM. CD4+ T cells are critical for corneal, but not skin, allograft rejection. Transplantation. 2000;69:483–487. doi: 10.1097/00007890-200002270-00004. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi M, Daniel V, Weimer R, Wiesel M, Hergesell O, Opelz G. Pre-transplant Th1 and post-transplant Th2 cytokine patterns are associated with early acute rejection in renal transplant recipients. Clin Transplant. 2003;17:151–157. doi: 10.1034/j.1399-0012.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 35.Chklovskaia E, Nowbakht P, Nissen C, Gratwohl A, Bargetzi M, Wodnar-Filipowicz A. Reconstitution of dendritic and natural killer-cell subsets after allogeneic stem cell transplantation: effects of endogenous flt3 ligand. Blood. 2004;103:3860–3868. doi: 10.1182/blood-2003-04-1200. [DOI] [PubMed] [Google Scholar]


