Abstract
Background
Bronchodilator response is seen in a significant proportion of patients with chronic obstructive pulmonary disease (COPD). However, there are also reports of a paradoxical response (PR) to beta2-agonists, resulting in bronchoconstriction. Asymptomatic bronchoconstriction is likely far more common but there has been no systematic study of this phenomenon.We assessed theprevalence of PR in current and former smokers with and without COPD, and its radiologic correlates and clinical implications.
Methods
Subjects from a large multicenter study (COPDGene) were categorized into two groups based on PR defined as at least a 12% and 200mLreduction in FEV1 and/or FVC after administration of a short-acting beta2-agonist (180ucg albuterol). Predictors of PR and associations with respiratory morbidity and computed tomographic measures of emphysema and airway disease were assessed.
Findings
9986 subjects were included. PR was seen in 4.54% and the frequency was similar in those with COPD and smokers without airflow obstruction. Compared to Caucasians, PR was twice as common in African-Americans (6.9% vs. 3.4%;p <0.001). On multivariate analyses, African- American race (adjusted OR 1.89, 95%CI 1.50 to 2.39), lesspercent emphysema (OR 0.96, 95%CI 0.92 to 0.99) and increased wall-area% of segmental airways (OR 1.04,95%CI 1.01 to 1.08) were independently associated with PR.PR was independently associated with worse dyspnea, lower six-minute-walk distance, higher BODE index, and a greater frequency of exacerbations(increased by a factor of 1.35, 95%CI 1.003 to 1.81).
Interpretation
Paradoxical response to beta2-agonists is associated with respiratory morbidity and is more common in African Americans.
Keywords: Paradoxical, bronchodilator, beta 2-agonist, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction that is not fully reversible.1However, improvements in lung function after short acting beta2-agonist and anticholinergic bronchodilators can be demonstrated in a significant proportion of patients with COPD.2There are also reports of a paradoxical response to beta2-agonists, resulting in bronchoconstriction and significant respiratory distress3-5 and it is likely that asymptomatic bronchoconstriction occurs more frequently6,7 though there has been no systematic study of this phenomenon. There are several potential reasons for a paradoxical response including an adverse reaction to either the bronchodilator itself or to inhaled preservatives or propellants. The patient characteristics underlying a paradoxical response and its clinical implications remain unknown. We hypothesized that a paradoxical response to beta2-agonistsin patients enrolled in the Genetic Epidemiology of COPD Study (COPDGene®)would beassociated with distinct radiologic phenotypes and with worse clinical outcomes.
Methods
Study design
Protocols for COPDGene subject enrollment and testing have been described previously.8 Briefly, non-Hispanic white and African-American current and former smokers aged 45 to 80 years were included in the study. Exclusion criteria included history of other lung diseases except asthma, prior lung surgery, pregnancy, active cancer undergoing treatment, and active or suspected lung cancer. Written informed consent was obtained from each subject and the COPDGene study was approved by the institutional review boards of all participating centers.
Spirometry
At study enrollment, each subject underwent pre- and post- bronchodilator spirometry using the ndd Easy-One spirometer (Andover, MA) before and 12-20 minutes afterinhalation of twopuffs of albuterol (90 mcg albuterol base per puff) with a spacer according to the American Thoracic Society (ATS) criteria.9Reference values were obtained from the National Health and Nutrition Examination Survey (NHANES) III data.10The diagnosis of COPD was made using a fixed post-bronchodilator cut-off of FEV1/FVC of <0.70.1 Details of spirometry procedures have been previously described.11
Bronchodilator response categorization
ATS criteria were adapted to define bronchodilator response.12Subjects were categorized into two groups based on a paradoxical response to beta 2-agonist (PR) defined as at least 12% and200 ml reduction in FEV1 and/or FVC. Percent reduction was assessed by [(postbronchodilator – prebronchodilator)/prebronchodilator] x 100. The volume criterion was included to account for a greater percent change in subjects with lower baseline lung function.12 As previous studies examining bronchodilator responses have mostly used percent criteria for FEV1, we also categorized subjects by 10% and 15% reduction in FEV1 alone (without FVC criteria) into PR10% and non-PR10%, and PR15% and non-PR15% groups.
Respiratory morbidity
The Modified Medical Research Council (MMRC) dyspnea score was used to quantify dyspnea 13 and the St George's Respiratory Questionnaire (SGRQ) scores to assess respiratory disease related health impairment and quality of life.14A standardized six minute walk test (6MWT) was performed according to ATS guidelines to assess functional capacity.8BODE (Body-Mass-Index, Airflow Obstruction, Dyspnea, and Exercise Capacity) Index was calculated to predict COPD-related mortality.15 Subjects were contacted every 3 to 6 months to obtain follow-up data on exacerbations, defined as episodes requiring use of either antibiotics and/or systemic steroids for acute worsening of respiratory symptoms, and severe exacerbations, defined as those requiring hospitalization.16
Imaging
Volumetric computed tomographic scans obtained at maximal inspiration (total lung capacity, TLC) and at end-tidal expiration (functional residual capacity, FRC) were analyzed for emphysema (% lung volume at TLC with attenuation less than -950 Hounsfield Units (HU), low attenuation area, %LAA950insp) and gas trapping (% lung volume at FRC with attenuation less than -856HU, %LAA856exp)using 3D Slicer software (www.airwayinspector.org).8 Emphysema was also assessed by Perc15, the lung density cut-off at which 15% of all voxels have a lower value.17Airway wall thickness was measured by quantitating wall area (WA%) of segmental bronchi and the Pi10 (square root of the airway wall area of a standardized airway of 10 mm luminal perimeter) using Pulmonary Workstation 2 (VIDA Diagnostics, Coralville, IA, USA).8
Statistical analyses
All values are expressed as mean (+standard deviation, SD). Univariate comparisons were made between PR+ and PR- groups using Chi-squared test for categorical variables, and 2-tailed independent t-test for continuous variables. Analyses were performed for the entire cohort and separately for subjects with COPD and smokers without airflow obstruction. Bivariate and multivariate linear regression models were created to assess the independent effects of PR status in the entire cohort on respiratory morbidity such as MMRC, SGRQ and 6MWT, with age, gender, race, smoking burden, current smoking status, FEV1, airway wall thickness and emphysema as covariates. Binary and multivariate logistic regression analyses were performed to assess the relationships betweenpatient demographics (such as age, race and gender), CT measures and FEV1, and PR status. Similar models were created for PR10%andPR15%. Differences in exacerbations and severe exacerbations over time were assessed using negative binomial regression models with age, gender, race, smoking status and FEV1 as covariates. No adjustments were made for multiple comparisons. P value <0.05 was considered statistically significant. For patients enrolled at the University of Alabama, general linear regression models were constructed to assess potential learning effects and fatigue associated with repeated efforts both pre- and post- bronchodilator. All analyses were performed using Statistical Package for the Social Sciences (SPSS 22.0, SPSS Inc., Chicago, IL, USA).
Role of the funding sources
The COPDGene study was funded by the National Institute of Health and ECLIPSE by GlaxoSmithKline.The sponsors of the two studieswere not involved in study design, data collection, data analysis, data interpretation, or writing of the report. SPB, YK and MTD had full access to the raw data, and the corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Demographics and Lung Function
Of 10,364 subjects enrolled in COPDGene, we excluded 64 who had significant interstitial lung disease or bronchiectasis, 108 normal controls, 143who did not have adequate spirometry data and 63 who had inadequate data for bronchodilator reversibility. Baseline characteristics of the final population for analysis (n=9986)are summarized in Table 1. The mean age of included subjects was 59.6 (SD 9) years; 4661 (46.7%) were female, 3282 (32.9%) were African American, and52.8% were current smokers. Physician-diagnosed asthma was reported by 17.6% of subjects. 4439 (44.5%) had COPD by GOLD criteria. Of these, 794 had GOLD (Global Initiative for Chronic Obstructive Lung Disease)spirometric severity classification grade I (17.7%), 1922 had grade II (42.9%), 1162 had grade III (25.9%), and 606(13.5%) had grade IV airflow obstruction. 1238 (12.4%) were GOLD unclassified,with reduced FEV1 (%predicted) but FEV1/FVC>0.70 (GOLD-U).
Table 1.
Baseline characteristics of patients
| All | COPD | Smokers | ||||
|---|---|---|---|---|---|---|
| PR+ (n=453) | Non-PR (n=9533) | PR+ (n=198) | Non-PR (n=4241) | PR+ (n=255) | Non-PR (n=5292) | |
| Demographics | ||||||
| Age (years) | 58.4 (8.9)** | 59.7 (9.0) | 62.2 (8.9) | 63.1 (8.6) | 55.5 (7.8)* | 56.9 (8.4) |
| Sex (%female) | 218 (48.1) | 4443 (46.6) | 77 (38.9) | 1885 (44.4) | 141 (55.3)* | 2558 (48.3) |
| Race (%African American) | 227 (50.1)† | 3055 (32.0) | 80 (40.4)† | 921 (21.7) | 147 (57.6)† | 2134 (40.3) |
| BMI (kg/m2) | 29.2 (6.5) | 28.8 (6.3) | 27.6 (6.1) | 27.9 (6.1) | 30.5 (6.6)* | 29.5 (6.3) |
| Pack-years | 45.5 (28.5) | 44.2 (24.7) | 53.1 (34.4) | 51.5 (26.8) | 39.5 (21.1.) | 38.4 (21.2) |
| Current smoker (%) | 289 (63.8)† | 4986 (52.3) | 102 (51.5)* | 1824 (43.0) | 187 (73.3)† | 3162 (59.8) |
| Asthma (%) | 75 (16.6) | 1679 (17.6) | 38 (19.2) | 964 (22.7) | 37 (14.5) | 715 (13.5) |
| Long acting antimuscarinic (%) | 82 (18.3) | 1582 (16.9) | 73 (37.6) | 1417 (34.3) | 9 (3.6) | 165 (3.2) |
| Long acting beta agonist (%) | 21 (4.7) | 330 (3.5) | 17 98.8) | 307 (7.5) | 4 (1.6)* | 23 (0.4) |
| Inhaled corticosteroids/Long acting beta agonist (%) | 86 (19.1) | 1841 (19.6) | 67 (34.2) | 1530 (36.8) | 19 (7.5) | 311 (6.0) |
| Spirometry | ||||||
| Pre-bronchodilator FEV1 (L) | 2.07 (0.86)* | 2.15 (0.92) | 1.57 (0.77) | 1.56 (0.77) | 2.45 (0.72)† | 2.63 (0.73) |
| Pre-bronchodilator FVC (L) | 3.22 (0.95) | 3.22 (1.03) | 2.96 (0.90) | 2.90 (1.03) | 3.43 (0.94) | 3.47 (0.95) |
| Pre-bronchodilator FEV1/FVC | 0.63(0.17)** | 0.65 (0.16) | 0.52 (0.18) | 0.52 (0.14) | 0.72 (0.09)† | 0.76 (0.06) |
| FEV1 %change | −7.6 (11.6)† | 6.3 (9.4) | −10.4 (13.4)† | 9.0 (11.3) | −5.4 (9.5)† | 4.1 (6.9) |
| FEV1 volume change (ml) | −173 (254)† | 105 (150) | −201 (272)† | 115 (144) | −151 (238)† | 97 (154) |
| FVC %change | −14.9 (9.1)† | 4.6 (10.3) | −12.8 (11.6)† | 8.8 (12.2) | −16.5 (5.9)† | 1.2 (6.9) |
| FVC volume change (ml) | −474 (321)f | 112 (269) | −361 (363)† | 215 (292) | −562 (252)† | 28 (2150 |
p<0.05.
p,0.01.
p<0.001
All values expressed as mean (SD), unless otherwise specified. PR = Paradoxical response to bronchodilator. BMI = Body Mass Index. FEVi = Forced Expiratory Volume in the first second. FVC = Forced Vital Capacity.
Paradoxical response (PR) was seen in 453 (4.54%) subjects. None of the subjects had symptomatic bronchoconstriction following administration of short acting beta2-agonist. Compared to Caucasians, African-Americans had twice the frequency of PR (6.9% vs. 3.4%; p <0.001). There was no difference between genders (4.4% in males and 4.7% in females; p = 0.53). The frequency of PR was similar in subjects with and without COPD (4.46% vs. 4.60%; p= 0.74) however, the frequency of PR increased with GOLD stage (1.8%,4.2%, 5.1%, and 7.4% respectively, from GOLD stages 1 to 4; p<0.001). Among healthy smokers, 1.3% exhibited PR due to FEV1 decline which was comparable to the frequency in those with GOLD stage 1 through 4 (2.4, 1.9, 1.2 and 3.2%, respectively). When PR was assessed by FVC decline, the rate was highest in those with GOLD 4 disease (6.5%) as compared to 3.2, 0.8, 2.1, and 3.9% in healthy smokers and GOLD stages 1 through 3 respectively. PR was noted in 8.6% of those with GOLD-U, of whom 0.7% had PR due to FEV1 decline and 7.8% had PR due to FVC decline.In the entire cohort, those with PR were younger, more likely to be African-American, more likely to be current smokers but with similar smoking burden, and had more severe airflow obstructionthan those without PR (Table 1). The frequency of physician-diagnosed asthma was similar in the two groups. We did not find any evidence that a learning effect or fatigue influenced the prevalence of PR in either the COPD or healthy smoker groups (Supplement Figure 1).
Medication use
There was no difference in the use of long-acting bronchodilators or inhaled steroids as maintenance therapy between the PR+ and PR- groups (Table 1). However, there was a significant difference in respiratory medication use between Caucasians and African-Americans as African-Americans with COPD were less likely to be on long acting anticholinergics (29.4% vs. 35.7%; p<0.001), long-acting beta agonists without concomitant inhaled steroids (5.6% vs. 8.0%; p=0.01) as well as any maintenance long-acting medication (42.4% vs. 50.8%;p<0.001). No directions were given for washout of inhalers prior to BDR testing, however the time of last inhalation was recorded for patients taking these medications and it was determined whether BDR testing was performed within or outside the recommended washout period (i.e. short acting beta-agonists 6 hours, long acting beta agonists 12 hours, etc.). There was no difference in the number of those who used an inhaler within the recommended washout period in the of PR+ and PR- patients (9.9% vs.8.8% for short acting beta agonists, 3.8% vs. 2.8% for long acting beta agonists, 14.1% vs. 15.2% for combined long acting beta agonists and inhaled corticosteroids, and 15.7% vs. 14.7% for long acting antimuscarinics; p not significant for all comparisons).
Imaging
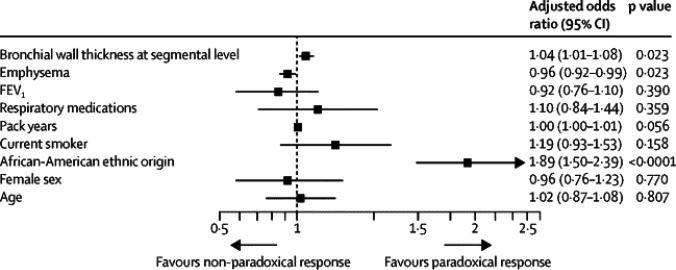
Table 2 summarizes the radiologic comparisons between the PR groups for the entire cohort and also by presence or absence of COPD. For the entire cohort, those with PR had thicker airway walls but less percent emphysema (Table 2). Total lung capacity by CT was lower in those with PR, but there was no difference in functional residual capacity. On multivariate analyses, African-American race (adjusted odds ratio, OR = 1.89, 95%CI 1.50 to 2.39), percent emphysema (OR0.96,95%CI 0.92 to 0.99) and segmental wall area% (OR = 1.04, 95%CI 1.01 to 1.08) were independently associated with PR (Figure 1). When paradoxical response was defined by %FEV1 reduction, African American race and segmental wall area% remained independentlyassociated with PR (Supplemental Tables 1 and 2).
Table 2.
Comparison of computed tomographic imaging and indices of respiratory morbidity
| All | COPD | Smokers | ||||
|---|---|---|---|---|---|---|
| PR+ (n=453) | Non-PR (n=9533) | PR+ (n=198) | Non-PR (n=4241) | PR+ (n=255) | Non-PR (n=5292) | |
| CT | ||||||
| TLC (L) | 5.2 (1.5)† | 5.5 (1.4) | 5.8 (1.5)* | 6.0 (1.4) | 4.6 (1.2)† | 5.2 (1.3) |
| FRC (L) | 3.3 (1.3) | 3.2 (1.1) | 4.0 (1.4) | 3.9 (1.2) | 2.7 (0.8) | 2.7 (0.7) |
| %LAA<-950insp | 5.5 (9.5) | 6.2 (9.6) | 10.6 (12.2) | 11.6 (12.2) | 1.5 (2.4)* | 1.9 (2.5) |
| Emphysema (Perc15) | −910.4 (34.3)† | −917.9 (31.6) | −930.9 (30.3)* | −935.8 (28.3) | −893.9 (27.7)† | −903.4 (26.2) |
| Gas trapping (%LAA<-856exp) | 22.9 (21.5) | 21.9 (19.9) | 37.7 (23.0) | 35.7 (20.7) | 11.2 (10.1) | 10.8 (9.6) |
| Wall Thickness (Wallarea% of segmental airways) | 62.1 (3.3)† | 61.4 (3.3) | 62.8 (3.2) | 62.4 (3.2) | 61.6 (3.2)† | 60.5 (3.1) |
| Pi10 | 3.71 (0.13)† | 3.68 (0.13) | 3.72 (0.14) | 3.70 (0.14) | 3.70 (0.12)† | 3.66 (0.12) |
| QoL | ||||||
| MMRC | 1.61 (1.48)† | 1.35 (1.45) | 2.05 (1.46) | 1.89 (1.47) | 1.27 (1.41)† | 0.92 (1.28) |
| 6MWD (m) | 381 (127)† | 413 (121) | 343 (127)† | 377 (124) | 410 (118)† | 442 (111) |
| SGRQ | 31.0 (23.2)† | 27.2 (22.9) | 38.8 (22.7) | 36.8 (22.9) | 25.0 (21.8)† | 19.5 (19.8) |
| BODE index | 1.87 (1.95)† | 1.43 (1.84) | 2.94 (2.12)** | 2.47 (2.11) | 1.04 (1.32)† | 0.62 (1.02) |
p<0.05.
p,0.01.
p<0.001
All values expressed as mean (SD), unless otherwise specified. PR = Paradoxical response to bronchodilator. TLC = Total Lung Capacity on computed tomography. FRC = Functional Residual Capacity on computed tomography. %LAA<950insp = %Low Attenuation Area below a threshold of −950 Hounsfield Units at end inspiration. Perc15 = the density of lung in HU below which 15% of the voxels had the lowest attenuation numbers at full inspiration. %LAA<856exp = %Low Attenuation Area below a threshold of −856 Hounsfield Units at end tidal expiration. Wallarea% = Bronchial wall area at segmental level. Pi10 = Square root of the airway wall area of a standardized airway of 10 mm luminal perimeter. MMRC = Modified Medical Research Council Dyspnea Scale. 6MWD = Six Minute Walk Distance. SQRQ = St. George's Respiratory Questionnaire.BODE = Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index.
Figure 1.
Multivariate logistic regression model showing adjusted odds ratios for predictors of Paradoxical Response. FEV1 = Pre-bronchodilator Forced Expiratory Volume in the first second. Perc15 = the density of lung in HU below which 15% of the voxels had the lowest attenuation numbers at full inspiration.Wallarea% = Bronchial wall area at segmental level. #Respiratory medications include one or more of inhaled long acting beta agonists, long acting antimuscarinic agents or a combination of inhaled corticosteroids/long acting beta agonists and theophylline. Odds Ratios depicted for every 10 HU change in Perc15, and for every 10 year change in age.
*p<0.05. †p<0.001
Respiratory outcomes
Respiratory morbidity was greater in those with PR as measured by a higher MMRC (1.61±1.48 vs. 1.35±1.45; p<0.001), higher SGRQ (31.0±23.2 vs. 27.2±22.9; p<0.001), and lower 6MWD (1250±416 vs. 1356±397 ft;p<0.001) (Table 1). PR status was also associated with a greater BODE index (1.87±1.95 vs. 1.43±1.84;p<0.001) (Table 1). On multivariate analysesadjusting for age, gender, race, smoking burden and smoking status, airflow obstruction, and CT indices of emphysema and airway wall thickness, PR was independently associated with a lower 6MWD and higher MMRC scores (Table 3). On univariate analysis, there was a significant association between PR and greater SGRQ scores, but this was not significant on multivariate analysis (Table 3). PR was also independently associated with a greater BODE index which in turn is a predictor of COPD related mortality (Table 3).
Table 3.
Univariate and Multivariate Associations between Paradoxical Response and respiratory morbidity
| Unadjusted beta regression co-efficient | 95% CI | Adjusted betaregression co-efficient | 95% CI | |
|---|---|---|---|---|
| 6MWD | ||||
| Age (years) | −7.02 † | −7.88 to −6.17 | −2.38 † | −3.40 to −1.36 |
| Female gender | −85.4 † | −101.0 to −69.8 | 33.9 † | 17.2 to 50.5 |
| African American Race | −130.5 † | −146.9 to −114.1 | −162.9 † | −179.9 to −146.0 |
| Current smoking | 0.91 † | 0.65 to 1.16 | −22.62 ** | −39.15 to −6.10 |
| Packyears | −3.27 † | −3.58 to −2.96 | −1.83 † | −2.13 to −1.54 |
| Pre-bronchodilator FEV1 | 213.2 † | 205.7 to 220.7 | 183.4 † | 171.7 to 195.2 |
| Emphysema (Perc 15) | 0.91 † | 0.65 to 1.16 | −0.21 | −0.48 to 0.06 |
| Wall Area% | −39.0 † | −41.3 to −36.7 | −14.0 † | −16.6 to −11.5 |
| PR | −106.3 † | −144.1 to −68.5 | −45.8 ** | −78.5 to −13.2 |
| R2 = 0.30 | ||||
| MMRC | ||||
| Age (years) | 0.01 † | 0.007 to 0.01 | −0.030 † | −0.033 to −0.026 |
| Female gender | 0.29 † | 0.23 to 0.35 | 0.20 † | 0.14 to 0.26 |
| African American Race | 0.19 † | 0.13 to 0.25 | −0.18 † | −0.24 to −0.12 |
| Current smoking | −0.09 ** | −0.15 to −0.03 | −0.04 | −0.10 to 0.02 |
| Packyears | 0.012 † | 0.011 to 0.013 | 0.007 † | 0.006 to 0.008 |
| Pre-bronchodilator FEV1 | −0.77 † | −0.80 to −0.75 | −0.80 † | −0.84 to −0.76 |
| Emphysema (Perc 15) | −0.007 † | −0.008 to −0.006 | −0.003 † | −0.004 to −0.002 |
| Wall Area% | 0.14 † | 0.13 to 0.15 | 0.034 † | 0.025 to 0.044 |
| PR | 0.26 † | 0.12 to 0.39 | 0.12* | 0.00 to 0.24 |
| R2 = 0.29 | ||||
| SGRQ | ||||
| Age (years) | 0.05 | −0.004 to 0.01 | −0.62 † | −0.68 to −0.56 |
| Female gender | 2.40 † | 1.50 to 3.29 | −5.92 † | −6.83 to −5.0 |
| African American Race | 2.37 † | 1.42 to 3.32 | 0.13 | −0.80 to 1.07 |
| Current smoking | 1.60 † | 0.71 to 2.50 | 2.47 † | 1.56 to 3.38 |
| Packyears | 0.23 † | 0.21 to 0.24 | 0.14 † | 0.12 to 0.16 |
| Pre-bronchodilator FEV1 | −12.4 † | −12.8 to −12.0 | −13.7 † | −14.4 to −13.1 |
| Emphysema (Perc 15) | −0.12 † | −0.13 to −0.10 | −0.05 † | −0.07 to −0.04 |
| Wall Area% | 2.52 † | 2.39 to 2.65 | 0.76 † | 0.62 to 0.90 |
| PR | 3.84 † | 1.68 to 6.0 | 1.32 | −0.47 to 3.12 |
| R2 = 0.35 | ||||
| BODE Index | ||||
| Age (years) | 0.033 † | 0.029 to 0.037 | −0.048 † | −0.052 to −0.044 |
| Female gender | 0.15 † | 0.08 to 0.23 | −0.70 † | −0.77 to −0.64 |
| African American Race | −0.04 | −0.12 to 0.03 | 0.30 | −0.03 to 0.09 |
| Current smoking | −0.44 † | −0.51 to −0.37 | −0.06 | −0.12 to 0.003 |
| Packyears | 0.018 † | 0.016 to 0.019 | 0.006 † | 0.005 to 0.007 |
| Pre-bronchodilator FEV1 | −1.36 † | −1.39 to −1.33 | −1.46 † | −1.50 to −1.41 |
| Emphysema (Perc 15) | −0.022 † | −0.023 to −0.021 | −0.012 † | −0.013 to −0.011 |
| Wall Area% | 0.20 † | 0.19 to 0.21 | 0.04 † | 0.03 to 0.05 |
| PR | 0.43 † | 0.26 to 0.61 | 0.31 † | 0.19 to 0.43 |
| R2 = 0.56 |
p<0.05.
p,0.01.
p<0.001
FEV1 = Forced Expiratory Volume in the first second. Perc15 = the density of lung in HU below which 15% of the voxels had the lowest attenuation numbers at full inspiration. Wallarea% = Bronchial wall area at segmental level. PR = Paradoxical response to beta2-agonists. BODE = Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index. All variables significant on bivariate analyses were entered into a multivariate model to assess independent associations.
For patients with COPD, PR status was also associated with a significantly higher risk of total exacerbations (an increase by a factor of 1.33, 95%CI 1.06 to 1.66; p=0.01) and severe exacerbations on follow up (an increase by a factor of1.35, 95%CI 1.003 to 1.81; p=0.048), after adjustment for age, race, gender, FEV1 and smoking status.
Discussion
Panel: Research in context
Systematic Review
We searched Pubmed and Google Scholar for publications related to “Paradoxical Response”, “Paradoxical bronchoconstriction”, “Paradoxical bronchospasm”, and “albuterol, bronchoconstriction” to identify relevant studies. We also identified pertinent references from the bibliographies of these publications. Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction that is not fully reversible. While bronchodilator responsiveness can be demonstrated in a significant proportion of patients with COPD, some patients have a paradoxical reduction in lung function following beta2-agonist administration. There has been no systematic study of this phenomenon.
Interpretation
This is the first systematic study describing the prevalence of a paradoxical response (PR) to beta2 agonists in smokers with and without COPD. This phenomenon is more common in African-Americans possibly explaining some of the poor outcomes seen in this population, and is independently associated with greater wall thickness and less emphysema on CT. PR is associated with worse respiratory outcomes including greater dyspnea and more frequent exacerbations.
We have demonstratedthat a paradoxical response to beta2-agonistsoccurs in smokers with and without airflow obstructionand that this phenomenonoccurs more frequently in AfricanAmericans and is associated with more severe airway disease and less percent emphysema as assessed by CT. This paradoxical response is also associated with respiratory morbidity including a greater risk of both moderate and severe COPD exacerbations.
Bronchodilator response (BDR) has long been assessed but its implications and clinical significance are unclear. Though lack of BDR has traditionally been deemed evidence of irreversible airflow obstruction,1 it is not specific in differentiating asthma from COPD7 anddoes not predict a therapeutic response to the regular administration of long acting bronchodilators.2 It is pertinent to note that BDR criteria are based on arbitrary spirometric thresholds and responses less than these cutoffs that might also have clinical implications. In this context, we explored the implications of a paradoxical worsening of lung function in response to beta2-agonists and found that this identifies a distinct subset of subjects with worse outcomes. Interestingly, all smokers seemed to be at risk for PR though its frequency tended to increase with increasing COPD severity.
In the absence of literature to guide us, we adapted the ATS criteria for BDR to define PR and used a 12% reduction in FEV1 or FVC as well as a 200 mL volume reduction.12 The latter was to account for those with more severe airflow obstruction who are expected to more easily meet PR criteria based solely on a percent change from baseline.19 It is generally accepted that a 100 mL change in FEV1 is clinically significant as this can often be perceived by patients.19 As these cut-offs remain somewhat arbitrary, we also defined PR using 10% and 15% thresholds for change in FEV120 and found similar predictors of PR.
The mechanisms underlying PR remain unknown. Acute bronchoconstriction following administration of bronchodilators has been described in case reports3-5andseveral mechanisms have been proposed including an adverse reaction to propellants used in metered dose inhalers,21 an immunoglobulin E mediated reaction to soy or lecithin containing excipients in the inhaler,22 as well as bronchial irritation due to preservatives or turbulent airflow resulting from inappropriate inhaler technique.21Preservatives such as sodium metabisulfite and benzalkonium chloride as well as hyperosmolality and acidity of the solutions have also been implicated in bronchoconstriction following nebulization of bronchodilators.23-25None of these reports were in patients with COPD. Previous use of bronchodilators can also lead to a lack of BDR due to down-regulation of receptors and development of airway subsensitivity,26but this is unlikely to lead to a paradoxical response. Subclinical bronchoconstriction is likely considerably more common than symptomatic bronchospasm, as evidenced by our study. We found a prevalence of PR much higher than the 0.24% reported in the UPLIFT (Understanding the Potential Long-Term Impacts on Function with Tiotropium) study7though this could be partly explained by the greater number ofAfrican-Americans enrolled in COPDGene and by the concurrent use of ipratropium for BDR testing in UPLIFT.It is not clear if adapting the UPLIFT protocol for bronchodilator responsiveness testing would have altered our findings.
Identification of PR has significant clinical implicationsasit is independently associated with worse respiratory outcomes.We found that PR status is not only associated with worse exercise tolerance and dyspnea, but is also a predictor of total and severe exacerbations. Paradoxical response was twice as common in African-Americans as in Caucasians. This is a novel finding that we speculate might explain some of the poor outcomes seen in African-Americans with COPDthat have often been explained by poor socioeconomic status,27 lower heath care utilization,28 and genetic susceptibility to greater airflow obstruction and emphysema for similar smoking burden.29 Though respiratory medication use was lower in African-Americans in our study suggesting disparities in care, this was not a predictor of PR status.Recent advances in genetics have also identified beta receptor polymorphisms as a potential source of differential response to beta agonists in African-Americans and our findings support the assertion that this is clinically relevant.30-32These findings imply that some subjects might do better with discontinuation of beta2-agonists, and further research is needed to test this hypothesis. Whether PR is modifiable or if it could be used to help select different therapies for this subset of patients is also unknown. Interestingly, we found a high frequency of PR in the GOLD-U group. This group is heterogeneous and is made up of patients with restrictive disorders as well as patients with less air trapping and bronchodilator responsiveness as well as thicker airway walls compared to patients with COPD.18 These characteristics would appear to predispose this population to a paradoxical response.
We found that PR is associated with distinct radiologic findings, though the differences in airway wall thickness and emphysema were modest. Increased airway thickness could be due to a combination of chronic bronchitis with mucus and smooth muscle hypertrophy33that could in turn be the result or cause of a poor response to bronchodilators. Increased airway thickness has been shown to correlate with symptoms independent of the degree of emphysema34,35and our results demonstrate that PR further predictspoor outcomes. We also found a weak inverse association between PR and the degree of emphysema. As flow limitation in COPD results from a combination of bronchoconstriction and decreased elastic recoil, we speculate that PR is more likely due to airway characteristics than reduced elastic recoil.
Our study has some limitations. We enrolled subjects who were current or former smokers, and thus our results might not be generalizable to populations at low risk for COPD. While there appear to be significant racial differences, the relatively small number of subjects with PR precluded meaningful genetic analyses. While learning effects and fatigue can potentially impact post bronchodilator values, these did not impact PR status in our study (Supplemental Figure 1). Subjects on long acting anti-muscarinics require a long washout period prior to bronchodilator reversibility testing which was not feasible in this study. Whilethe lack of instructions for medication withholding may have introduced bias, there was no difference in maintenance medication use between those with and without PR nor any difference in the number of subjects who used an inhaler during the washout period between these two groups. CT scans were not spirometrically gated which can affect measurements of emphysema, however detailed instructions were given to subjects to maximize the probability of obtaining scans at full inspiration (TLC) and at end expiration. Finally, we did aim to validate these findings by examining data from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort.36 However we found a lower rate of PR [47 (1.9%) of the 2493 patients], perhaps due to the low number of individuals of African descent enrolled (44 of the 2493), and this precluded meaningful analysis. Additional studies are needed to determine if PR+ is a clinically stable phenotypeand to confirm if it is a feature that identifies a set of African Americans with specific clinical features.
In summary, we demonstrate that PR is more common in African Americans and is associated with significantly worse respiratory outcomes.
Supplementary Material
Acknowledgments
Funding Source:
COPDGene is supported by NIH Grant Numbers R01 HL089897 and R01 HL089856.ECLIPSE was funded by GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Study design: SPB and MTD
Statistical analyses: SPB and YK
Data interpretation: SPB, JMW, VK, GJC, CPH, MH, WCB, HN, YK, MGF, DSS, CGW, SIR, EKS, BJM and MTD
Manuscript writing: SB, MTD
Critical review of the manuscript for important intellectual content: SPB, JMW, VK, GJC, CPH, MH, WCB, HN, YK, MGF, DSS, CGW, SIR, EKS, BJM and MTD
Declaration of Interest:
SPB, GJC, MH, YK, MGF, DSS andCGWhave no conflicts of interest relevant to this manuscript to declare. JMW declares research contracts from GSK and Forest, outside the submitted work. VK reports grants from NHLBI, personal fees from CSA Medical, personal fees from Medscape, other from Boehringer Ingelheim, other from GlaxoSmithKline, other from MedImmune, outside the submitted work. EKS reports grants from NIH, grants and travel expenses from COPD Foundation, during the conduct of the study; grants and personal fees from GlaxoSmithKline, personal fees from Merck, and travel expenses from Novartis, outside the submitted work. HN reports grants from COPDGene, during the conduct of the study; other from Altria, other from Philip Morris Intl, outside the submitted work. CPH reports grants from National Institutes of Health, during the conduct of the study; personal fees from Novartis, personal fees from CSL Behring, outside the submitted work. BJM reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants, non-financial support and other from AstraZeneca, other from Forest, grants, personal fees and other from Boehringer Ingelheim, grants, personal fees and other from Pfizer, other from Respironics, other from Spiration, grants, personal fees and other from GlaxoSmithKline, grants from Nabi, grants and personal fees from Sunovian, grants from National Heart, Lung, and Blood Institute, other from Merck, other from Ikaria, other from Coviden, other from Consensus Medical Education, other from Cedars Sinai Medical Center, other from Integrity Medical Education, other from Mt. Sinai Medical Center, other from Carden Jennings, other from WebMD, other from Aerocrine, other from Up-To-Date, other from Theravance, other from Foundation for Improving Patient Outcomes, outside the submitted work.MTD reports grants from NHLBI, during the conduct of the study; grants from NHLBI, grants from Aeris, grants from AstraZeneca, grants and personal fees from Boston Scientific, grants and personal fees from Boerhinger Ingelheim, grants and personal fees from GlaxoSmithKline, personal fees from Ikaria, grants from Pulmonx, grants from PneumoRx, grants from Otsuka, grants from Pearl, grants from Forest, grants from American Heart Association, outside the submitted work. WCB reports personal fees from DLA Piper, outside the submitted work.
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. The lancet Respiratory medicine. 2013;1(7):564–73. doi: 10.1016/S2213-2600(13)70086-9. [DOI] [PubMed] [Google Scholar]
- 3.Broski SE, Amundson DE. Paradoxical response to levalbuterol. J Am Osteopath Assoc. 2008;108(4):211–3. [PubMed] [Google Scholar]
- 4.Raghunathan K, Nagajothi N. Paradoxical bronchospasm: a potentially life threatening adverse effect of albuterol. South Med J. 2006;99(3):288–9. doi: 10.1097/01.smj.0000202699.93002.e8. [DOI] [PubMed] [Google Scholar]
- 5.Spooner LM, Olin JL. Paradoxical bronchoconstriction with albuterol administered by metered-dose inhaler and nebulizer solution. Ann Pharmacother. 2005;39(11):1924–7. doi: 10.1345/aph.1G248. [DOI] [PubMed] [Google Scholar]
- 6.Hodder R, Pavia D, Lee A, Bateman E. Lack of paradoxical bronchoconstriction after administration of tiotropium via Respimat(R) Soft Mist Inhaler in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:245–51. doi: 10.2147/COPD.S16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. The European respiratory journal. 2008;31(4):742–50. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 8.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt SP, Kim YI, Wells JM, et al. FEV/FEV to Diagnose Airflow Obstruction: Comparisons with Computed Tomography and Morbidity Indices. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201308-251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–6. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 14.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. The American review of respiratory disease. 1992;145(6):1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 15.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. The New England journal of medicine. 2004;350(10):1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JI, Moyle S, Criner GJ, et al. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. Copd. 2012;9(5):466–72. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170(11):1172–8. doi: 10.1164/rccm.200406-761OC. [DOI] [PubMed] [Google Scholar]
- 18.Wan ES, Hokanson JE, Murphy JR, et al. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184(1):57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–69. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 20.Criteria for the assessment of reversibility in airways obstruction. Report of the Committee on Emphysema American College of Chest Physicians. Chest. 1974;65(5):552–3. doi: 10.1378/chest.65.5.552. [DOI] [PubMed] [Google Scholar]
- 21.Nicklas RA. Paradoxical bronchospasm associated with the use of inhaled beta agonists. J Allergy Clin Immunol. 1990;85(5):959–64. doi: 10.1016/0091-6749(90)90084-h. [DOI] [PubMed] [Google Scholar]
- 22.Facchini G, Antonicelli L, Cinti B, Bonifazi F, Massei V. Paradoxical bronchospasm and cutaneous rash after metered-dose inhaled bronchodilators. Monaldi Arch Chest Dis. 1996;51(3):201–3. [PubMed] [Google Scholar]
- 23.Cocchetto DM, Sykes RS, Spector S. Paradoxical bronchospasm after use of inhalation aerosols: a review of the literature. J Asthma. 1991;28(1):49–53. doi: 10.3109/02770909109073370. [DOI] [PubMed] [Google Scholar]
- 24.Finnerty JP, Howarth PH. Paradoxical bronchoconstriction with nebulized albuterol but not with terbutaline. Am Rev Respir Dis. 1993;148(2):512–3. doi: 10.1164/ajrccm/148.2.512. [DOI] [PubMed] [Google Scholar]
- 25.O'Callaghan C, Milner AD, Swarbrick A. Paradoxical deterioration in lung function after nebulised salbutamol in wheezy infants. Lancet. 1986;2(8521-22):1424–5. doi: 10.1016/s0140-6736(86)92735-2. [DOI] [PubMed] [Google Scholar]
- 26.Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet. 1995;346(8969):201–6. doi: 10.1016/s0140-6736(95)91265-7. [DOI] [PubMed] [Google Scholar]
- 27.Dransfield MT, Bailey WC. COPD: racial disparities in susceptibility, treatment, and outcomes. Clin Chest Med. 2006;27(3):463–71. vii. doi: 10.1016/j.ccm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Shaya FT, Maneval MS, Gbarayor CM, et al. Burden of COPD, asthma, and concomitant COPD and asthma among adults: racial disparities in a medicaid population. Chest. 2009;136(2):405–11. doi: 10.1378/chest.08-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(6):1110–6. doi: 10.1016/j.rmed.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Elbahlawan L, Binaei S, Christensen ML, Zhang Q, Quasney MW, Dahmer MK. Beta2-adrenergic receptor polymorphisms in African American children with status asthmaticus. Pediatr Crit Care Med. 2006;7(1):15–8. doi: 10.1097/01.pcc.0000194010.63115.a2. [DOI] [PubMed] [Google Scholar]
- 31.Naqvi M, Thyne S, Choudhry S, et al. Ethnic-specific differences in bronchodilator responsiveness among African Americans, Puerto Ricans, and Mexicans with asthma. J Asthma. 2007;44(8):639–48. doi: 10.1080/02770900701554441. [DOI] [PubMed] [Google Scholar]
- 32.Naqvi M, Tcheurekdjian H, DeBoard JA, et al. Inhaled corticosteroids and augmented bronchodilator responsiveness in Latino and African American asthmatic patients. Ann Allergy Asthma Immunol. 2008;100(6):551–7. doi: 10.1016/S1081-1206(10)60055-5. [DOI] [PubMed] [Google Scholar]
- 33.Patel BD, Coxson HO, Pillai SG, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2008;178(5):500–5. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 34.Mair G, Maclay J, Miller JJ, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med. 2010;104(11):1683–90. doi: 10.1016/j.rmed.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Martinez CH, Chen YH, Westgate PM, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.