Abstract
Purpose of review
To describe the role of the magnesium transporter 1 (MAGT1) in the pathogenesis of “X-linked immunodeficiency with magnesium defect, Epstein-Barr virus (EBV) infection, and neoplasia” (XMEN) disease and its clinical implications.
Recent findings
The magnesium transporter protein MAGT1 participates in intracellular Mg2+ homeostasis and facilitates a transient Mg2+ influx induced by activation of the T cell receptor (TCR). Loss-of-function mutations in MAGT1 cause an immunodeficiency named XMEN syndrome characterized by CD4 lymphopenia, chronic EBV infection, and EBV-related lymphoproliferative disorders. Patients with XMEN disease have impaired T cell activation and decreased cytolytic function of natural killer (NK) and CD8+ T cells due to decreased expression of the natural killer stimulatory receptor natural-killer group 2, member D (NKG2D). Patients may have defective specific antibody responses secondary to T cell dysfunction, but B cells have not been shown to be directly affected by mutations in MAGT1.
Summary
XMEN disease has revealed a novel role for free intracellular magnesium in the immune system. Further understanding of the MAGT1 signaling pathway may lead to new diagnostic and therapeutic approaches.
Keywords: immunodeficiency, MAGT1, NKG2D, chronic Epstein-Barr virus infection, EBV-associated lymphoma, Mg2+ supplementation
INTRODUCTION
Magnesium ion (Mg2+) is the most abundant divalent cation in eukaryotic cells and required for many critical physiological processes. The vast majority of the total body’s Mg2+ content is in a bound form either in the intracellular compartment or in bone. Most intracellular bound Mg2+ is tightly associated with nucleotides and proteins and plays vital roles in energy production, DNA replication, gene transcription and protein synthesis (1). Less than 5% of the intracellular Mg2+ exists in the free (ionized) state for which magnesium transporter 1 (MAGT1) is a critical regulator (2).
Loss-of-function mutations in the gene encoding MAGT1 cause XMEN disease, a mild form of combined immune deficiency (CID) that has brought to light novel and fascinating functions of free intracellular Mg2+ in the immune system. We now know that a transient T cell receptor (TCR) mediated Mg2+ influx into T cells is a signaling step that enhances the efficiency of the activation of these cells (3). Intracellular Mg2+ regulation in the immune system participates in the cytolytic control of EBV infection (4). An important connection between Mg2+ and EBV control was recognized in patients with XMEN disease in whom a decrease in intracellular free Mg2+ was associated with defective expression of the natural killer stimulatory receptor NKG2D in natural killer (NK) and cytotoxic CD8+ T lymphocytes (CTL). As a result, XMEN patients have high levels of EBV and an increased number of EBV-infected B cells in the blood. XMEN disease is also associated with an increased susceptibility to EBV-driven lymphoproliferative diseases. Moreover, recent in-vitro and preliminary in-vivo studies using oral magnesium L-threonate supplementation in XMEN patients showed increases in NKG2D on endogenous NK and CTL cells, which led to better control of EBV infection (4).
In recent years, several primary immunodeficiencies have thus been associated with abnormalities in ion channels and transporters, including those involved in the permeability and/or transport of Ca2+ (5–8), Mg2+ (3, 4, 9, 10), and Zn2+ ions (11–13). This review highlights the most up-to-date evidence on the clinical and pathophysiologic features of XMEN disease, a new X-linked genetic disorder with abnormal Mg2+ transportation, chronic EBV infection, increased susceptibility to EBV-associated B cell lymphoma, decreased CD4 count, and impaired T cell and NKG2D-driven cytolytic function caused by loss-of-function mutations in MAGT1.
CLINICAL FEATURES
CIDs are a heterogeneous group of genetic abnormalities that impair both humoral and cell-mediated immunity. In contradistinction to severe combined immunodeficiency (SCID) where, if left untreated, patients usually succumb to severe infections within the first year or two of life, XMEN disease has a more indolent clinical course. EBV-associated B cell lymphoproliferation ultimately emerges in late childhood to be the most common cause of severe morbidity and mortality in this patient population. The prevalence of XMEN disease is unknown, but it is expected to be uncommon. Only 7 male patients with XMEN disease have been reported in the scientific literature and the frequency of female carriers has not yet been defined (9). More recently, a lower number of unpublished male cases have been identified. Because the association between MAGT1 loss of function mutations and immune disease was not recognized until 2011, increased awareness of XMEN disease in the medical community might lead to identification of new cases, especially in male patients with a history of persistently elevated EBV viral loads and/or EBV-associated lymphoproliferative diseases.
In our experience at the National Institutes of Health, the age at diagnosis varies from 3 to 45 years (9). Recurrent sinopulmonary infections and viral pneumonias have been reported in some but not all patients with XMEN disease. Interestingly, EBV-associated lymphomas have been the only reason some patients with XMEN disease come to medical attention. Because XMEN is an X-linked disease, all patients with clinical disease to date have been males. Women with verified mutations in the gene that encodes MAGT1 have all been unaffected heterozygous carriers with skewed lyonization favoring expression of the wild type X chromosome. As in other X-linked diseases, there is a theoretical possibility of clinical disease due to homozygous MAGT1 mutations in females from an inbred population, an abnormally skewed female carrier towards the mutant X chromosome, or even less likely, the coexistence of a MAGT1 mutation and an X chromosome monosomy (Turner’s syndrome) in the same individual.
The major clinical features of XMEN disease include persistent elevation in EBV-viral load, EBV-associated lymphoproliferative disorders, often with splenomegaly, dysgammaglobulinemia, and decreased CD4/CD8 ratio. The most common clinical characteristic among all patients with XMEN has been a persistent elevation in EBV viral load with an associated increased susceptibility to EBV-driven B cell lymphomas/lymphoproliferative disorders (3). The amount of EBV virus in peripheral blood in XMEN disease also varies among individuals, with values ranging from a thousand to millions of copies per milliliter as determined by quantitative real-time polymerase chain reaction (PCR). All post-pubertal XMEN patients in our cohort have developed at least one episode of EBV-associated B cell lymphoproliferative disease; two individuals have evidence of two independent primary lymphomas. Two patients aged 23 and 45 years died of transplant-related complications shortly after undergoing hematopoietic stem cell transplantation.
Additionally, XMEN patients may have susceptibility to sinopulmonary and ear infections, viral pneumonias, and other viral infections, but these are generally mild or infrequent. Cutaneous viral infections such as molluscum contagiosum (MCV) have only been reported in one XMEN child. However, MCV is not confined to PID patients, and it is estimated that approximately less than 5% of children in the United States show clinical evidence of MCV infection indicating the XMEN association may be coincidental (14). Human-papilloma virus (HPV) infections are not commonly observed. Severe varicella infection followed by recurrent herpes zoster was reported in one patient. Neither clinically significant fungal infections nor persistent cytomegalovirus infection (CMV) have been observed. Mild elevations in liver transaminases with no other associated gastrointestinal symptoms or abnormalities have been transiently seen in some patients, likely secondary to chronic EBV infection. Autoimmune cytopenias are not a cardinal feature, but autoimmune hemolytic anemia, thrombocytopenia, and neutropenia have been reported in two patients.
Patients with XMEN have normal growth and development, and no evidence of mental retardation has been identified in any of these patients. These findings are consistent with recent work that found that previous implications of MAGT1 in mental retardation to be unsupported (15). Failure to thrive – a common feature in SCID – has not been reported in XMEN disease.
XMEN disease shares some similarities with other PIDs, including those characterized by increased susceptibility to EBV infections, an oncogenic B cell-tropic gammaherpesvirus that is widespread and infects the majority of children and adults worldwide(16). EBV is the main causative agent of asymptomatic toddler infections and infectious mononucleosis in teenagers, but generally persists as a latent life-long infection in most adults, and has been associated with T, B, and Hodgkin’s lymphomas as well as nasopharyngeal carcinoma. The autosomal recessive interleukin-2 inducible tyrosine kinase (ITK) deficiency is also characterized by EBV-associated lymphoproliferation (17–20). However, ITK deficiency has a more severe clinical course of immune dysregulation and rapid disease progression compared to XMEN. CD4 lymphopenia can be seen in both XMEN and a subgroup of patients with ITK deficiency (19), but decreased number of invariant natural killer T (iNKT) cells —a hallmark of ITK deficiency—is not seen in XMEN disease. Absent or low iNKT cells in the setting of persistent EBV infection are also seen in X-linked lymphoproliferative (XLP) disease due to deficiency of SLAM associated protein (SAP) or X-linked inhibitor of apoptosis protein (XIAP). The latter was previously considered a form of XLP (XLP2) but has more recently been classified as an X-linked familial hemophagocytic lymphohistiocytosis (21, 22). Fulminant infectious mononucleosis (FIM), the most common clinical manifestation of XLP has not been observed in XMEN disease. Hemophagocytic lymphohistiocytosis (HLH), a common EBV-triggered complication in XIAP, rarely occurs in XMEN. We have only observed EBV-positive lymphoproliferative diseases in XMEN. Persistent symptomatic EBV viremia is seen in CD27 deficiency, a form of combined immunodeficiency associated with hypogammaglobulinemia and impairment in specific-antibody function resulting from disturbed CD8+ T cell and T cell dependent B cell responses (23). However, the level of EBV viremia in the two cases with CD27 deficiency reported to date has been lower (range <50–234 copies /mL) than what is usually seen in XMEN. Chronic EBV viremia with or without associated persistent cytomegalovirus (CMV) viremia has been described in individuals with a p110δ-activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency (PASLI) disease, also known as activated PI3K-delta syndrome (APDS) (24, 25). In contrast to XMEN, a major hallmark of PASLI is the presence of lymphoid nodules on the respiratory and gastrointestinal tracts and a shift of naïve to senescent T cells. Other primary immunodeficiencies characterized by increased susceptibility to EBV-associated lymphoproliferation include coronin-1A deficiency (26), and MST1 deficiency (27, 28)
GENETICS
XMEN disease is caused by loss-of-function mutations in MAGT1. In humans, the MAGT1 gene is located on chromosome Xq21.1. MAGT1 is a 10-exon gene that may have multiple in-frame translation initiation sites. It encodes the transmembrane (TM) protein MAGT1, an evolutionary conserved, magnesium-specific transporter protein with little similarity to any known transporter except TUSC3, a non-selective Mg2+ transporter and candidate tumor suppressor gene originally named N33 and located on the chromosomal band 8p22(29, 30). The human MAGT1 has a 66% amino acid sequence homology with the human TUSC3 and both genes have similar predicted secondary structures. The full-length MAGT1 protein has 367 amino acids, a large amino-terminal segment, 4 TM domains, and a small carboxy-terminal tail (29, 30). Mutations in TUSC3 have been associated with nonsyndromic autosomal recessive retardation (31–34), prostate cancer (35), and ovarian cancer (36, 37), which have not been observed in XMEN disease.
Several different MAGT1 mutations have been identified in patients with XMEN. In all cases, the resulting changes in the DNA sequence have lead to premature translational termination with nonsense-mediated decay of the messenger ribonucleic acid (mRNA) (3). In our experience, the time to onset of lymphoma in XMEN cannot be predicted by the MAGT1 genotype alone. Whether the age of first exposure to EBV or other environmental factors plays a role in the clinical presentation remains to be determined.
Normal females transcriptionally silence one of their two X chromosomes in each somatic cell, a process called X chromosome inactivation (XCI) or lyonization. The pattern of XCI in somatic progenitor cells is transmitted to its progeny in a highly stable manner. In some PIDs, such as X-linked chronic granulomatous disease (CGD), the normal and abnormal population of phagocytes coexists in female carriers. Thus, one cell population will express the wild type CYBB, the causative gene of X-linked CGD, and have normal superoxide production, while the other population will carry the affected allele, yielding the characteristic mosaic pattern with oxidative testing (38). In other X-linked PIDs, the abnormal allele interferes with normal cell development, and the affected cell line in female carriers will be predominantly comprised of cells bearing the wild type gene. As an example, carrier females with mutations in the Bruton tyrosine kinase (BTK) gene that causes X-linked agammaglobulinemia (XLA) have only one population of B cells, deriving from precursor B cells that express the normal BTK allele due to skewing of survival of B cells having inactivated the X chromosome harboring the defective BTK allele (39, 40). Because of the small number of XMEN cases reported to date, only a few female carriers have undergone XCI analysis. Notably, the mother and grandmother of two XMEN patients have completely skewed XCI favoring expression of the wild type X chromosome only in the hematopoietic lineages and not in epithelial cells (3). This suggests the possibility of a selective developmental disadvantage in hematopoietic cells or their progenitors expressing only the mutant MAGT1 allele.
PATHOGENESIS
MAGT1 is ubiquitously expressed in all mammalian cells. It is a highly selective Mg2+ transporter with little permeability to other divalent cations (41). MAGT1 is a cell surface (plasma membrane) protein that governs the balance of Mg2+ between the extracellular fluid and the intracellular free basal pool (29). In T and B cells, MAGT1 participates in intracellular Mg2+ homeostasis (4, 42), and its expression level is upregulated when extracellular Mg2+ is low (29). Patients with XMEN have normal bound intracellular Mg2+ but the free (ionized) basal magnesium, as well as the transient TCR-gated Mg2+ flux, is defective in XMEN (3).
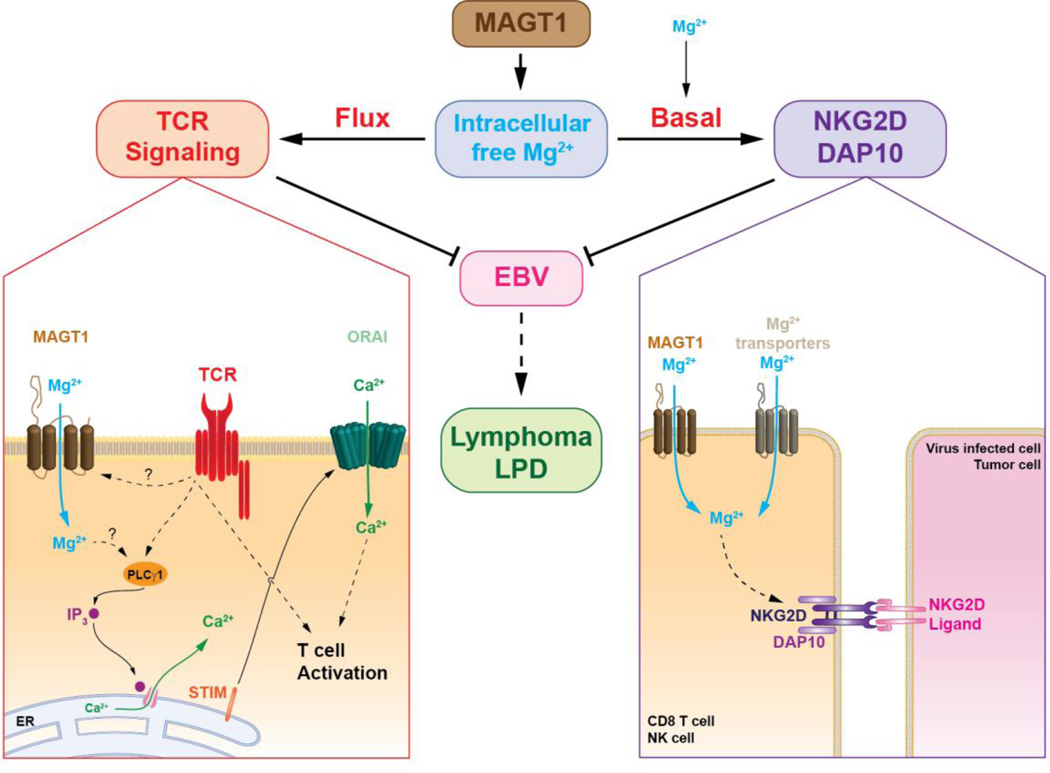
The loss of MAGT1 causes impaired T cell activation due to abolition of the rapidly induced and transient TCR-driven Mg2+ flux. This leads to delayed phosphorylation of phospholipase Cγ1 (PLCγ1) and decreased downstream generation of calcium (Ca2+) flux (3) (Figure 1). The mechanisms by which TCR stimulation induces a MAGT1 mediated transient Mg2+influx into T cells and how this rapid increase in the free intracellular Mg2+ concentration activates PLCγ1 have not been determined yet. Although B cells express MAGT1, BCR stimulation does not induce a Mg2+ flux. Remarkably, in contrast to T cells, Ca2+ flux is intact in XMEN B cells. B cells rely on PLCγ2 for the B cell receptor (BCR)-induced Ca2+ influx, which appears to be a MAGT1-independent process (3).
Figure 1.
Magnesium transporter 1 (MAGT1) regulates free basal magnesium concentrations and is responsible for the rapid and transient magnesium flux after T cell receptor (TCR) stimulation. In normal T cells, stimulation of the TCR triggers a transient Mg2+flux through MAGT1. This rapid increase of intracellular Mg2+is required for the activation of phospholipase Cγ1 (PLCγ1) required for the downstream generation of Ca2+ flux through the Ca2+ channel ORAI by promoting the release of Ca2+ from the endoplasmic reticulum (ER) via inositol 1,4,5-triphosphate (IP3) and its receptor, leading to activation of the stromal interaction molecule (STIM) and its translocation into the ER-plasma membrane junctions where they activate ORAI as shown in the TCR signaling diagram (left). The absence of MAGT1 leads to the chronic reduction of intracellular free Mg2+, which is required to maintain the expression of NKG2D (shown associated with adaptor molecules DAP10) and the normal cytolytic function of NK and CD8+ T cells as shown in the NKG2D/DAP10 diagram. Loss of MAGT1 results in defective TCR signaling and decreased expression of NKG2D. These two defects lead to failure to clear EBV infection and increased susceptibility to EBV-positive lymphomas/lymphoproliferative disease (LPD).
Another consequence of a loss-of-function in MAGT1 is a chronic decrease in the basal level of intracellular free magnesium, leading to reduced expression of the activator receptor NKG2D on NK cells and CD8+ T cells (3, 4, 9) (Figure 1). NKG2D has important functions in the cytolytic control of EBV-infected cells and tumor surveillance. XMEN is the first PID associated with decreased expression of NKG2D. Impaired NGK2D function may account for the persistent EBV viremia and increased susceptibility to EBV-driven lymphoproliferative diseases seen in XMEN even though these patients are capable of generating EBV-specific cytotoxic T lymphocytes in vivo (4). Importantly, in vitro studies of XMEN patients’ cells show that supplementation of the tissue culture medium with magnesium sulfate (MgSO4) leads to a partial but significant recovery of NKG2D expression and better cytotoxic function in both NK and CD8+ T cells (4). This has also been observed in vivo in XMEN patients receiving oral supplementation with high dose magnesium L-threonate (4).
Finally, MAGT1 is homologous to a subunit of yeast oligosaccharyl transferase (OST) and its precise cellular functions have not been fully determined. A recent study suggests that MAGT1 can also serve as an endoplasmic reticulum (ER)-localized subunit of the OST complex involved in N-glycosylation of specific glycoproteins (43). Further investigation is required to determine the MAGT1 role in glycosylation in healthy and diseased lymphocytes.
DIAGNOSIS AND TREATMENT
XMEN disease should be suspected in male individuals with unexplained chronic, especially high level, elevations in EBV viremia, splenomegaly, and a personal or family history of EBV-positive B cell lymphoproliferative diseases. EBV serologies may show evidence of past infection. EBV DNA PCR is used to determine and follow-up EBV viremia in XMEN. Measurements for this assay vary widely from center to center, but this should be improved with the implementation of World Health Organization (WHO) international standards in the near future. Quantification of EBV-infected B cells by flow cytometric fluorescence in situ hybridization (FISH) is a novel technique used in some research laboratories and will show elevated values in XMEN patients. XMEN patients may present with an inverted CD4:CD8 ratio and a reduced number of CD31+ cells in their naïve CD4+ T cell population, suggesting decreased thymic output (3, 9). Additionally, XMEN is associated with B cell lymphocytosis due to chronic EBV infection (9). Mild transaminase elevations, CD4 lymphopenia, impaired T-cell lymphoproliferation upon TCR stimulation, and dysgammaglobulinemia (with or without clinical evidence of humoral dysfunction) are other clinical features that may be associated with XMEN disease (9). When available, testing for a decreased NKG2D expression in CD8+ T cells and NK cells, as detected by flow cytometry on whole blood samples, strongly supports the diagnosis of XMEN disease. Downregulation of NKG2D has also been described in the setting of malignancies (44), and confirmatory genetic testing for MAGT1 should be pursued. In the United States, MAGT1 exon sequencing can be currently performed through the Cincinnati Children’s Molecular Genetics Laboratory or through the National Institutes of Health Clinical Center. To rule out potential non-coding mutations affecting MAGT1 expression, we recommend studying the MAGT1 mRNA expression if no mutations are found and the clinical suspicion remains high. Quantification of the intracellular free Mg2+ flux in T cells after TCR stimulation is a research test not currently available for clinical use. Total serum magnesium levels in patients with XMEN are usually within the normal range and are not useful for the diagnosis of XMEN.
Treatment of disease manifestations and prophylactic therapy in XMEN depends on the individual clinical features. Hypogammaglobulinemia or impaired response to vaccines may be treated with immunoglobulin replacement therapy or antibiotic prophylaxis, similar to other humoral PIDs. Antiviral prophylaxis may also be considered on an individual basis. Chemotherapy is reserved for treatment of malignancies in XMEN. We have observed that oral Mg2+ supplementation restores the basal intracellular free magnesium, leading to increased NKG2D expression on CD8+ T and NK cells, which correlates with a decrease in EBV viremia and the percentage of EBV-infected cells in XMEN patients in vivo (4). To date, treatment with oral Mg2+ L-threonate has shown to be a safe and well-tolerated therapeutic modality in XMEN. Diarrhea, a common adverse effect seen with some oral Mg2+ formulations, has not been a major complaint. Because the serum Mg2+ concentration is regulated by the renal system, this treatment modality should not be used in patients with impaired renal function. Other contraindications for Mg2+ treatment include hypersensitivity to any component of the formulation, and cardiac abnormalities such as certain types of heart block. We have not seen any clinically significant cardiovascular or neuromuscular adverse effects in XMEN patients treated with Mg2+ L-threonate. Although Mg2+ supplementation has shown promising results in XMEN, it is not yet known whether this is sufficient to prevent EBV-driven lymphoproliferative complications. Ablative B cell therapy with the anti-CD20 antibody rituximab has been successfully used in a few XLP patients to control acute EBV infection and prevent FIM (45). However, rituximab does not completely deplete CD20+ B cells in tissues (46), and does not prevent the development of EBV-positive CD20− lymphoproliferative lesions (47). Autologous virus-specific T cell therapy may also be used, but it is unlikely to completely overcome the impaired EBV-specific killing defect seen in T cells in XMEN disease (4). Matched third-party EBV-specific T cells may be a therapeutic option in XMEN. Although the only two XMEN patients who underwent hematopoietic stem cell transplantation (HSCT) for treatment of EBV-positive tumors died of transplant-related complications, HSCT may still be potentially curative and could be considered for patients with biopsy-proven lymphoproliferative malignancies or for those who are in remission after successful chemotherapy (9). Given the small number of XMEN cases reported to date, the natural history of the disease is not well known, which complicates making firm therapeutic considerations regarding HSCT, especially in patients who have no clinical or laboratory evidence of an ongoing lymphoproliferative process.
CONCLUSIONS
XMEN disease has revealed a novel role for free intracellular magnesium regulation in the immune system. XMEN patients do not suffer from overwhelming infections as seen in SCID or other CIDs, which is consistent with the mild T-cell activation defect in these individuals. XMEN has been defined only recently and the long-term natural history of disease is currently uncertain. Thus far, the morbidity associated with XMEN disease appears to result mainly from failure of the immune system to control EBV infection, which invariably leads to EBV-driven malignancies. Given the role for NKG2D in tumor surveillance, it is unknown if acquisition of EBV infection is necessary for the development of malignancies in XMEN. Mg2+ supplementation safely increases the basal intracellular Mg2+ pool in XMEN lymphocytes and NK cells, increasing NKG2D expression and ultimately decreasing EBV viremia. Thus, this therapeutic approach may prove beneficial in the long-term for XMEN. Increased awareness of this disease in the medical community may help identify new XMEN cases and increase our understanding of the natural history of this disease. Further clinical trials will be necessary to explore therapeutic options in XMEN.
KEYPOINTS.
MAGT1 is a critical regulator of intracellular free Mg2+ in the immune system.
Loss-of-function mutations in MAGT1 abolish the transient TCR-induced Mg2+ flux that is essential for optimal T-cell activation.
The chronic decrease in free basal Mg2+ in XMEN leads to decreased expression of NKG2D, resulting in impaired clearance of EBV-infected cells and increased susceptibility to EBV-driven lymphoproliferative complications.
Mg2+ supplementation may reduce the number of EBV-infected cells and potentially decrease the risk of developing lymphoma in XMEN disease.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH and by co-funding through the Office of Disease Prevention, NIH. We thank Dr. Helen Su for critically reading the manuscript; Drs. William Paul, Kathryn Zoon, and Anthony Fauci for their special support of the NIAID Clinical Genomics program.
Footnotes
Conflicts-of-interest disclosure: The authors declare no competing financial interests.
References and recommended reading
Papers of particular interest, published in the years 2013 and 2014, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wolf FI, Trapani V. Cell (patho)physiology of magnesium. Clinical science. 2008;114(1):27–35. doi: 10.1042/CS20070129. [DOI] [PubMed] [Google Scholar]
- 2.Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6(3):113–127. [PubMed] [Google Scholar]
- 3.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaigne-Delalande B, Li FY, O'Connor GM, Lukacs MJ, Jiang P, Zheng L, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341(6142):186–191. doi: 10.1126/science.1240094. ••This study shows the critical role of MAGT1 in regulating free basal intracellular Mg2+concentrations and demonstrates a link between NKG2D cytolytic activity and EBV antiviral immunity in humans.
- 5.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 6.Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. The New England journal of medicine. 2009;360(19):1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. The Journal of allergy and clinical immunology. 2009;124(6):1311–1318. e7. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske S, Picard C, Fischer A. Immunodeficiency due to mutations in ORAI1 and STIM1. Clinical immunology. 2010;135(2):169–182. doi: 10.1016/j.clim.2010.01.011. This stu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li FY, Chaigne-Delalande B, Su H, Uzel G, Matthews H, Lenardo MJ. XMEN disease: a new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood. 2014;123(14):2148–2152. doi: 10.1182/blood-2013-11-538686. ••Comprehensive review of XMEN disease, including clinical manifestations, genotypic features, pathogenic mechanisms, diagnostic and therapeutic strategies for XMEN.
- 10.Li FY, Lenardo MJ, Chaigne-Delalande B. Loss of MAGT1 abrogates the Mg2+ flux required for T cell signaling and leads to a novel human primary immunodeficiency. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2011;24(3):S109–S114. doi: 10.1684/mrh.2011.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nature genetics. 2002;31(3):239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. American journal of human genetics. 2002;71(1):66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaigne-Delalande B, Lenardo MJ. Divalent cation signaling in immune cells. Trends in immunology. 2014;35(7):332–344. doi: 10.1016/j.it.2014.05.001. ••Detailed review of the roles of Ca2+, Mg2+, and Zn2+ as second messengers in the immune system.
- 14.Dohil MA, Lin P, Lee J, Lucky AW, Paller AS, Eichenfield LF. The epidemiology of molluscum contagiosum in children. Journal of the American Academy of Dermatology. 2006;54(1):47–54. doi: 10.1016/j.jaad.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 15. Piton A, Redin C, Mandel JL. XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. American journal of human genetics. 2013;93(2):368–383. doi: 10.1016/j.ajhg.2013.06.013. •Re-evaluation of the genetic causes of X-linked intellectual disability using new genomic methods.
- 16.Cohen JI. Epstein-Barr virus infection. The New England journal of medicine. 2000;343(7):481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 17.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. The Journal of clinical investigation. 2009;119(5):1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linka RM, Risse SL, Bienemann K, Werner M, Linka Y, Krux F, et al. Loss-of-function mutations within the IL-2 inducible kinase ITK in patients with EBV-associated lymphoproliferative diseases. Leukemia. 2012;26(5):963–971. doi: 10.1038/leu.2011.371. [DOI] [PubMed] [Google Scholar]
- 19.Serwas NK, Cagdas D, Ban SA, Bienemann K, Salzer E, Tezcan I, et al. Identification of ITK deficiency as a novel genetic cause of idiopathic CD4+ T-cell lymphopenia. Blood. 2014;124(4):655–657. doi: 10.1182/blood-2014-03-564930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stepensky P, Weintraub M, Yanir A, Revel-Vilk S, Krux F, Huck K, et al. IL-2-inducible T-cell kinase deficiency: clinical presentation and therapeutic approach. Haematologica. 2011;96(3):472–476. doi: 10.3324/haematol.2010.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filipovich AH, Zhang K, Snow AL, Marsh RA. X-linked lymphoproliferative syndromes: brothers or distant cousins? Blood. 2010;116(18):3398–3408. doi: 10.1182/blood-2010-03-275909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh RA, Madden L, Kitchen BJ, Mody R, McClimon B, Jordan MB, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116(7):1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Montfrans JM, Hoepelman AI, Otto S, van Gijn M, van de Corput L, de Weger RA, et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. The Journal of allergy and clinical immunology. 2012;129(3):787–793. e6. doi: 10.1016/j.jaci.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nature immunology. 2014;15(1):88–97. doi: 10.1038/ni.2771. ••The authors describe a group of patients with p110δ-activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency (PASLI) disease, a combined immunodeficiency and lymphoproliferative disease caused by gain-of-function mutations inPIK3CD.This work showed that treatment with sirolimus to inhibit mTOR activity in vivo partially rescued the in vitro T cell defects and improved the clinical course.
- 25. Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–871. doi: 10.1126/science.1243292. ••Remarkable description of patients from several unrelated families affected with a dominant gain-of-function mutation in PIK3CD.
- 26.Moshous D, Martin E, Carpentier W, Lim A, Callebaut I, Canioni D, et al. Whole-exome sequencing identifies Coronin-1A deficiency in 3 siblings with immunodeficiency and EBV-associated B-cell lymphoproliferation. The Journal of allergy and clinical immunology. 2013;131(6):1594–1603. doi: 10.1182/blood-2012-07-440339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schaffer AA, et al. The phenotype of human STK4 deficiency. Blood. 2012;119(15):3450–3457. doi: 10.1182/blood-2011-09-378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nehme NT, Pachlopnik Schmid J, Debeurme F, Andre-Schmutz I, Lim A, Nitschke P, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. 2012;119(15):3458–3468. doi: 10.1182/blood-2011-09-378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. American journal of physiology Cell physiology. 2010;298(3):C407–C429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 31.Garshasbi M, Hadavi V, Habibi H, Kahrizi K, Kariminejad R, Behjati F, et al. A defect in the TUSC3 gene is associated with autosomal recessive mental retardation. American journal of human genetics. 2008;82(5):1158–1164. doi: 10.1016/j.ajhg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garshasbi M, Kahrizi K, Hosseini M, Nouri Vahid L, Falah M, Hemmati S, et al. A novel nonsense mutation in TUSC3 is responsible for non-syndromic autosomal recessive mental retardation in a consanguineous Iranian family. American journal of medical genetics Part A. 2011;155A(8):1976–1980. doi: 10.1002/ajmg.a.34077. [DOI] [PubMed] [Google Scholar]
- 33.Khan MA, Rafiq MA, Noor A, Ali N, Ali G, Vincent JB, et al. A novel deletion mutation in the TUSC3 gene in a consanguineous Pakistani family with autosomal recessive nonsyndromic intellectual disability. BMC medical genetics. 2011;12:56. doi: 10.1186/1471-2350-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molinari F, Foulquier F, Tarpey PS, Morelle W, Boissel S, Teague J, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. American journal of human genetics. 2008;82(5):1150–1157. doi: 10.1016/j.ajhg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horak P, Tomasich E, Vanhara P, Kratochvilova K, Anees M, Marhold M, et al. TUSC3 loss alters the ER stress response and accelerates prostate cancer growth in vivo. Scientific reports. 2014;4:3739. doi: 10.1038/srep03739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pils D, Horak P, Vanhara P, Anees M, Petz M, Alfanz A, et al. Methylation status of TUSC3 is a prognostic factor in ovarian cancer. Cancer. 2013;119(5):946–954. doi: 10.1002/cncr.27850. [DOI] [PubMed] [Google Scholar]
- 37.Vanhara P, Horak P, Pils D, Anees M, Petz M, Gregor W, et al. Loss of the oligosaccharyl transferase subunit TUSC3 promotes proliferation and migration of ovarian cancer cells. International journal of oncology. 2013;42(4):1383–1389. doi: 10.3892/ijo.2013.1824. [DOI] [PubMed] [Google Scholar]
- 38.Holland SM. Chronic granulomatous disease. Hematology/oncology clinics of North America. 2013;27(1):89–99. viii. doi: 10.1016/j.hoc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkelstein JA, Fearon E. Carrier detection of the X-linked primary immunodeficiency diseases using X-chromosome inactivation analysis. The Journal of allergy and clinical immunology. 1990;85(6):1090–1097. doi: 10.1016/0091-6749(90)90055-9. [DOI] [PubMed] [Google Scholar]
- 40.Conley ME, Brown P, Pickard AR, Buckley RH, Miller DS, Raskind WH, et al. Expression of the gene defect in X-linked agammaglobulinemia. The New England journal of medicine. 1986;315(9):564–567. doi: 10.1056/NEJM198608283150907. [DOI] [PubMed] [Google Scholar]
- 41.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deason-Towne F, Perraud AL, Schmitz C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS letters. 2011;585(14):2275–2278. doi: 10.1016/j.febslet.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. The Journal of cell biology. 2014;206(4):525–539. doi: 10.1083/jcb.201404083. ••The authors describe the role of MagT1 in N-linked glycosylation of specific glycoproteins in the endoplasmic reticulum of HeLa cells.
- 44.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 45.Milone MC, Tsai DE, Hodinka RL, Silverman LB, Malbran A, Wasik MA, et al. Treatment of primary Epstein-Barr virus infection in patients with X-linked lymphoproliferative disease using B-cell-directed therapy. Blood. 2005;105(3):994–996. doi: 10.1182/blood-2004-07-2965. [DOI] [PubMed] [Google Scholar]
- 46.Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney allotransplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(10):2418–2428. doi: 10.1111/j.1600-6143.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- 47.Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117(22):5835–5849. doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]