Abstract
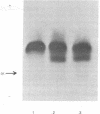
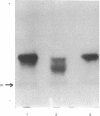
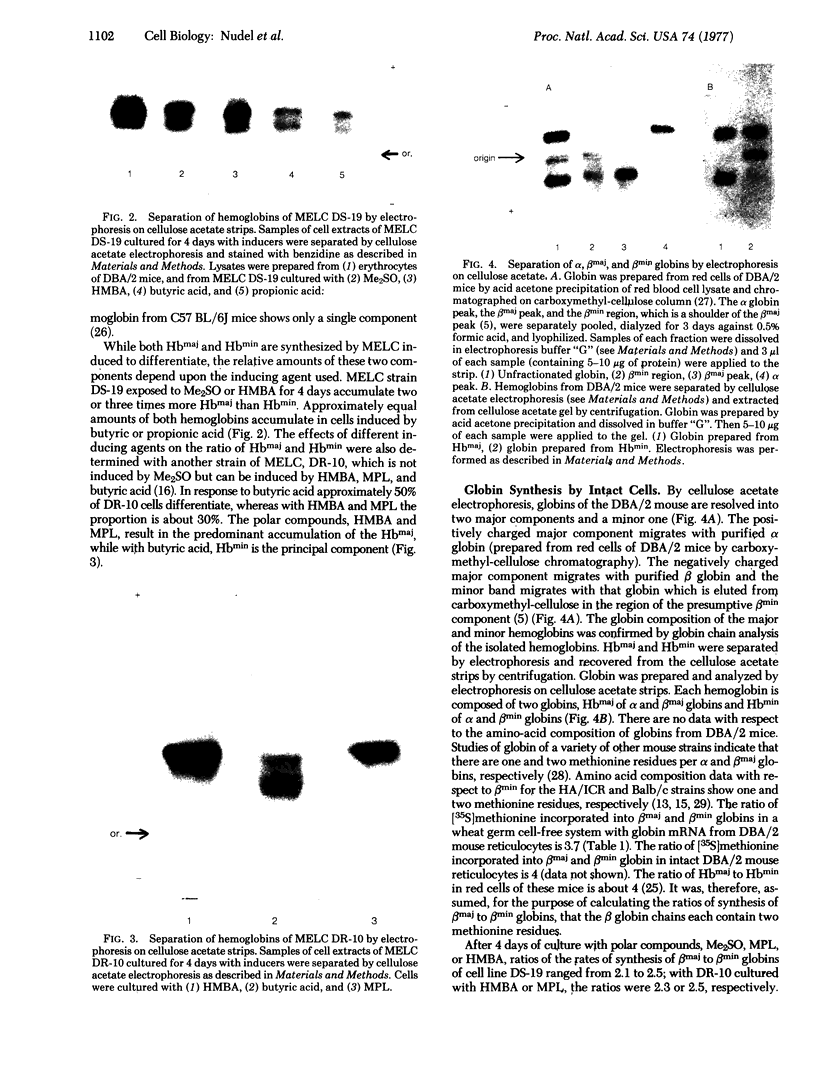
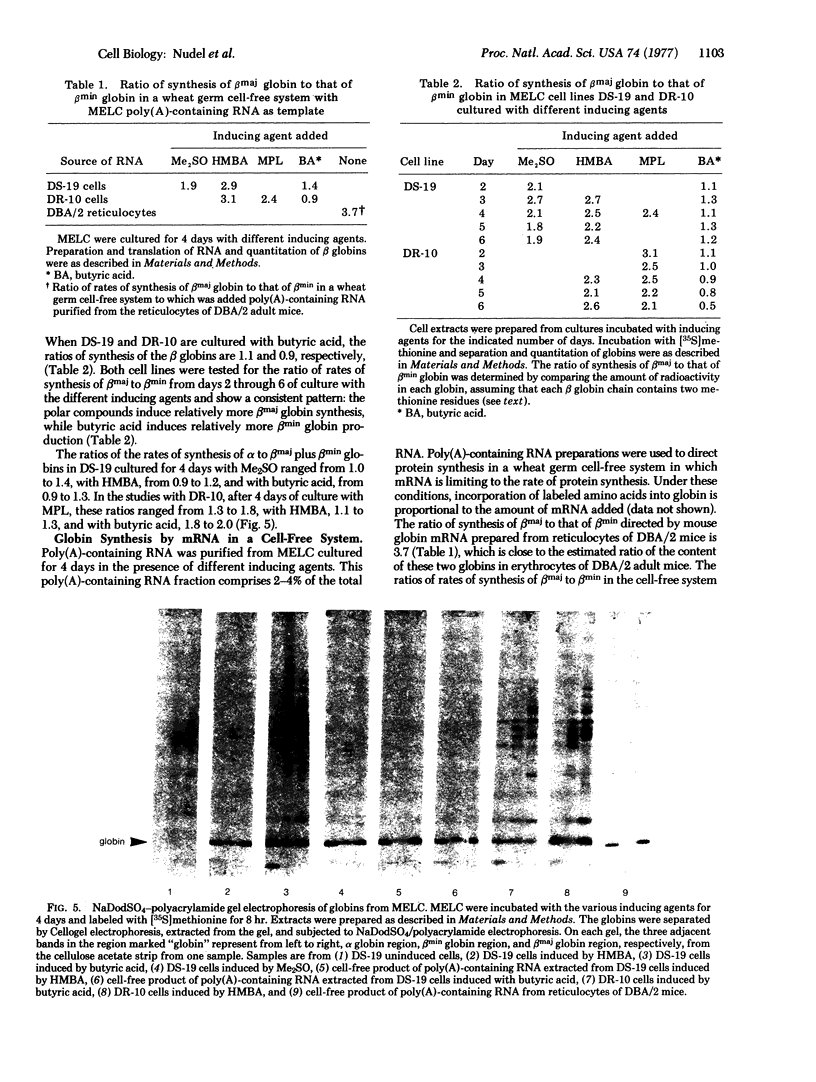
Murine erythroleukemia cells are induced to erythrodifferentiate by polar compounds such as dimethyl sulfoxide and hexamethylene bisacetamide as well as by fatty acids such as butyric acid and propionic acid. The effect of these inducers on the expression of two beta globin genes, betamaj and betamin, during the course of differentiation of the cells has been examined. After 4 days of culture with hexamethylene bisacetamide or dimethyl sulfoxide, the betamaj-containing hemoglobin (Hbmaj) predominates. By contrast, in the presence of butyric acid or propionic acid, after 4 days of culture, relatively equal amounts of Hbmaj and Hbmin are found. When cultured with dimethyl sulfoxide or hexamethylene bisacetamide, murine erythroleukemia cells synthesize more betamaj than betamin, while about equal amounts of the two globins are synthesized in the presence of butyric acid. When poly(A)-containing RNA from the cells exposed to different inducers is translated in a wheat germ cell-free system, the ratio of betamaj to betamin synthesized reflects that in whole cells. In a strain of murine erythroleukemia cells resistant to dimethyl sulfoxide (DR-10), the preferential stimulation of betamaj synthesis by hexamethylene bisacetamide of the betamin synthesis by butyric acid is more pronounced than with the dimethyl sulfoxide-sensitive cells (DS-19). These data suggest that polar compounds and fatty acids cause different expression of the betamaj and betamin genes in murine erythroleukemia cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Wuu K. D., Noyes A. N., Young R., Scher W., Friend C., Preisler H. D., Bank A. Hemoglobin biosynthesis in murine virus-induced leukemic cells in vitro: structure and amounts of globin chains produced. Blood. 1972 Dec;40(6):823–835. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G. Hemoglobin Beta chain structural variation in mice: evolutionary and functional implications. Science. 1972 Nov 24;178(4063):873–874. doi: 10.1126/science.178.4063.873. [DOI] [PubMed] [Google Scholar]
- Gilman J. G. Mouse haemoglobin Beta chains. Sequence data on embryonic y chain and genetic linkage of the Y-chain locus to the adult beta-chain locus Hbb. Biochem J. 1976 May 1;155(2):231–241. doi: 10.1042/bj1550231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G. Mouse haemoglobin beta chains. Comparative sequence data on adult major and minor beta chains from two species, Mus musculus and Mus cervicolor. Biochem J. 1976 Oct 1;159(1):43–53. doi: 10.1042/bj1590043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J. F., Housman D. Induction of erythroid differentiation in vitro by purines and purine analogues. Cell. 1976 Jun;8(2):263–269. doi: 10.1016/0092-8674(76)90010-6. [DOI] [PubMed] [Google Scholar]
- HUTTON J. J., BISHOP J., SCHWEET R., RUSSELL E. S. Hemoglobin inheritance in inbred mouse strains. I. Structural differences. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1505–1513. doi: 10.1073/pnas.48.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Furusawa M., Sugano H. Erythrocyte membrane-specific antigens in Friend virus-induced leukemia cells. Bibl Haematol. 1973;39:955–967. doi: 10.1159/000427928. [DOI] [PubMed] [Google Scholar]
- Kabat D., Sherton C. C., Evans L. H., Bigley R., Koler R. D. Synthesis of erythrocyte-specific proteins in cultured friend leukemia cells. Cell. 1975 Jul;5(3):331–338. doi: 10.1016/0092-8674(75)90109-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Tanaka M., Terada M., Miller O. J., Bank A., Marks P., Rifkind R. A. Erythroid cell differentiation: murine erythroleukemia cell variant with unique pattern of induction by polar compounds. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1232–1236. doi: 10.1073/pnas.73.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G. Sequence of amino acids in the major and minor chains of the diffuse hemoglobin from BALB-c mice. Biochim Biophys Acta. 1973 Mar 23;303(1):61–67. doi: 10.1016/0005-2795(73)90148-7. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Ikawa Y., Leder P. Globin messenger-RNA induction during erythroid differentiation of cultured leukemia cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3620–3623. doi: 10.1073/pnas.69.12.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Singer D., Cooper M., Maniatis G. M., Marks P. A., Rifkind R. A. Erythropoietic differentiation in colonies of cells transformed by Friend virus. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2668–2670. doi: 10.1073/pnas.71.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Levy J., Terada M., Breslow R., Rifkind R. A., Marks P. A. Induction of erythroid differentiation in murine virus infected eythroleukemia cells by highly polar compounds. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1003–1006. doi: 10.1073/pnas.72.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S., Schneider R. G. Rapid differentiation of polypeptide chains of hemoglobin by cellulose acetate electrophoresis of hemolylsates. Blood. 1969 Aug;34(2):230–235. [PubMed] [Google Scholar]