Abstract
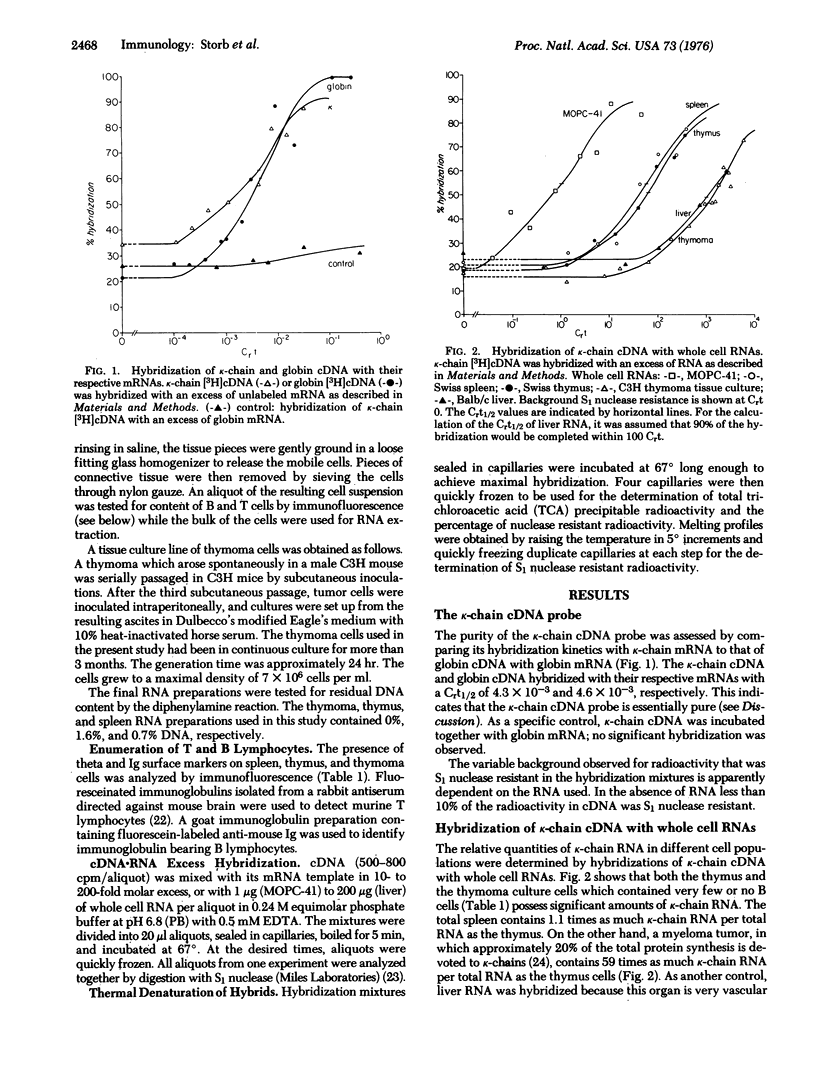
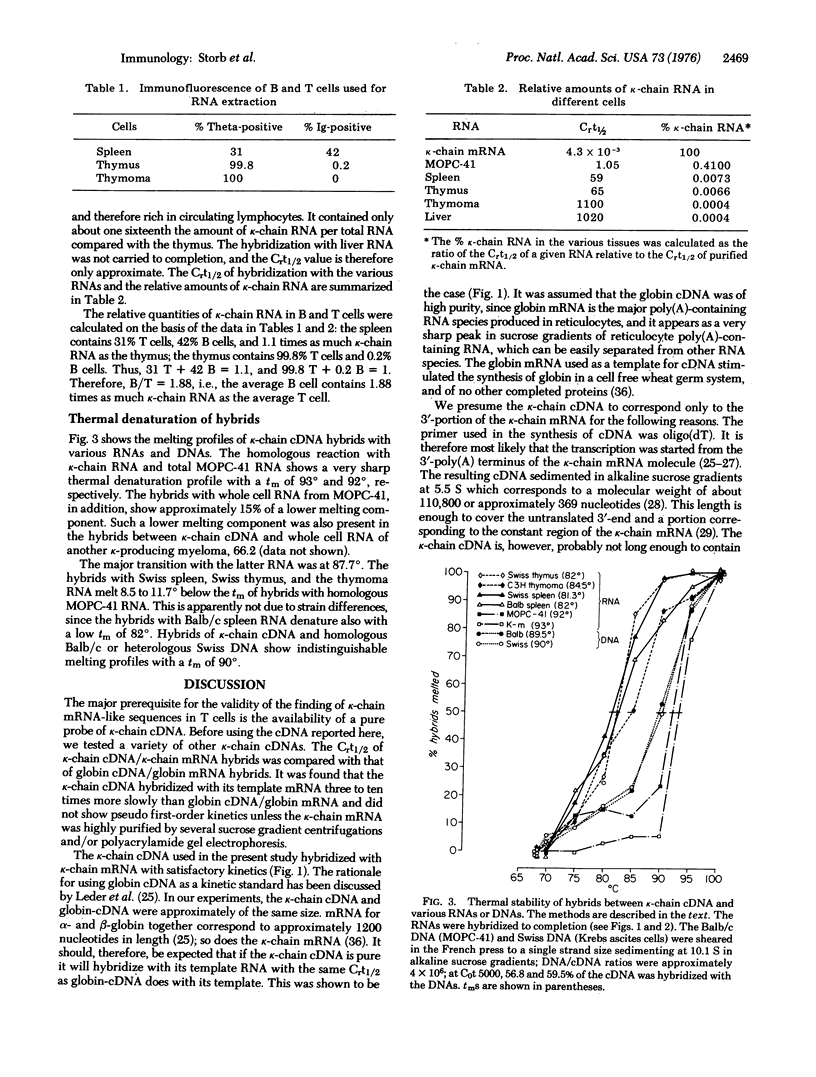
We investigated by molecular hybridization whether T cells contain RNA sequences homologous to RNA which codes for immunoglobulin kappa-chain (k-chain). A radioactive probe of complementary DNA (cDNA) was prepared by transcription of purified k-chain mRNA from mouse myeloma MOPC-41 with reverse transcriptase (RNA-dependent-DNA nucleotidyltransferase) from avian myeloblastosis virus. The cDNA probably corresponded only to the constant region and 3'-terminus of k-chain mRNA. Kappa-chain cDNA was found to hybridize efficiently with RNA from both thymus cells and an established culture of thymoma cells. The thymus and thymoma cells contained 99.8% and 100% theta-positive cells, respectively. Quantitatively the average thymus T cell (thymus derived lymphocyte) contained about one half as much k-chain mRNA as the average spleen B cell ("bursa" dependent lymphocyte), whereas the thymoma cells contained only 1/33 as much. Control hybridizations of k-chain cDNA with myeloma and liver RNA support the conclusion that T cells in the thymus and in the thymoma cell line synthesize k-chain mRNA-like molecules. The thermal stability of hybrids of k-chain cDNA with RNA from spleen, thymus, thymoma, and another k-chain producing myeloma tumor was lower than that with MOPC-41 RNA. This finding may be due to the existence of several slightly different ck genes in the mouse as suggested by various control experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLTON E. T., MCCARTHY B. J. FRACTIONATION OF COMPLEMENTARY RNA. J Mol Biol. 1964 Feb;8:201–209. doi: 10.1016/s0022-2836(64)80129-7. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Boylston A. W. Theta antigen and immunogolbulin on a tissue-cultured mouse lymphoma. Immunology. 1973 May;24(5):851–857. [PMC free article] [PubMed] [Google Scholar]
- Faust C. H., Diggelmann H., Mach B. Estimation of the number of genes coding for the constant part of the mouse immunoglobulin kappa light chain. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2491–2495. doi: 10.1073/pnas.71.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W., Deutsch H. F. The variability of human lambda-chain constant regions and some relationships to V-regions sequences. Immunochemistry. 1976 Feb;13(2):149–155. doi: 10.1016/0019-2791(76)90283-4. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Cogen R. B. Immunoglobulin molecules on the surface of activated T lymphocytes in the rat. J Exp Med. 1973 Jul 1;138(1):163–175. doi: 10.1084/jem.138.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Colón S., Campbell P., Rabellino E. Immunoglobulins on the surface of lymphocytes. V. Quantitative studies on the question of whether immunoglobulins are associated with T cells in the mouse. J Immunol. 1972 Oct;109(4):776–783. [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Honjo T., Packman S., Swan D., Nau M., Norman B. The organization and diversity of immunoglobulin genes. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5109–5115. doi: 10.1073/pnas.71.12.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Rinuy A., Vassalli P. Absence of detectable IgM in enzymatically or biosynthetically labeled thymus-derived lymphocytes. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2879–2883. doi: 10.1073/pnas.70.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Atwell J. L., Cone R. E. Isolation of surface immunoglobulin from lymphocytes from human and murine thymus. Nat New Biol. 1972 Feb 23;235(60):240–242. doi: 10.1038/newbio235240a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Cartwright E. M., Jarvis J. M., Proudfoot N. J. Sequence analysis of immunoglobulin light chain messenger RNA. Nature. 1974 Nov 29;252(5482):354–359. doi: 10.1038/252354a0. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz C., Hahn Y. Cell-surface immunoglobulin human thymus cells and its biosynthesis in vitro. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3716–3720. doi: 10.1073/pnas.70.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz C., Lahat N. Surface immunoglobulin of mouse thymus cells and its in vitro biosynthesis. Cell Immunol. 1974 Sep;13(3):397–406. doi: 10.1016/0008-8749(74)90259-7. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Miller J. F., Forni L., Sprent J. Immunoglobulin on activated T cells detected by indirect immunofluorescence. Cell Immunol. 1974 Mar 15;10(3):476–482. doi: 10.1016/0008-8749(74)90139-7. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Hyman R., Epstein R., Nakoinz I., Cohn M. Independence of theta and TL surface antigens and killing by thymidine, cortisol, phytohemagglutinin, and cyclic AMP in a murine lymphoma. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1085–1091. doi: 10.1016/s0006-291x(73)80006-3. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C., Stavnezer E., Bishop J. M. Isolation of messenger RNA for an immunoglobulin kappa chain and enumeration of the genes for the constatn region of kappa chain in the mouse. J Mol Biol. 1974 Sep 5;88(1):43–63. doi: 10.1016/0022-2836(74)90294-0. [DOI] [PubMed] [Google Scholar]
- Storb U. Quantitation of immunoglobulin genes by nucleic acid hybridization with RNA from myeloma and spleen microsomes. J Immunol. 1972 Mar;108(3):755–764. [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Bianco C., Nussenzweig V., Uhr J. W. Cell surface immunoglobulin. IV. Distribution among thymocytes, bone mrrow cells, and their derived populations. J Exp Med. 1972 Jul 1;136(1):81–93. doi: 10.1084/jem.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Boyse E. A. Immunoglobulin synthesis and secretion by cells in the mouse thymus that do not bear theta antigen. Proc Natl Acad Sci U S A. 1973 Mar;70(3):834–838. doi: 10.1073/pnas.70.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Warner N. L. Membrane immunoglobulins and antigen receptors on B and T lymphocytes. Adv Immunol. 1974;19(0):67–216. doi: 10.1016/s0065-2776(08)60252-7. [DOI] [PubMed] [Google Scholar]



