Abstract
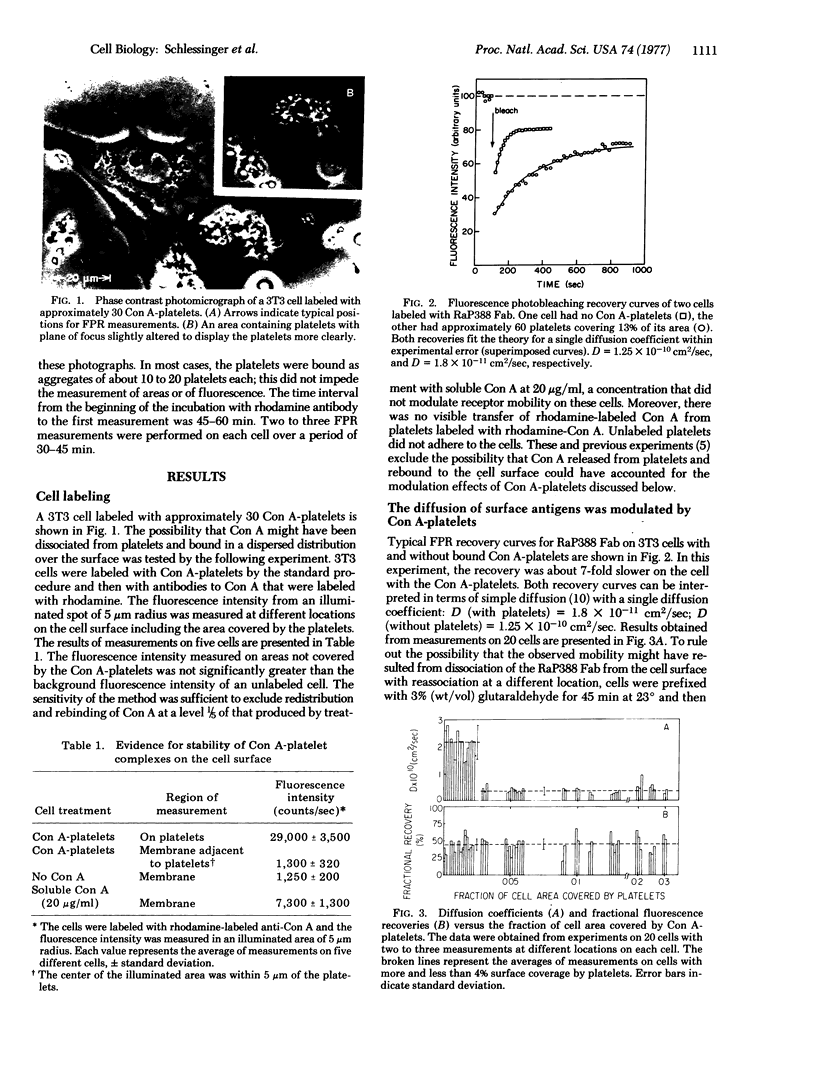
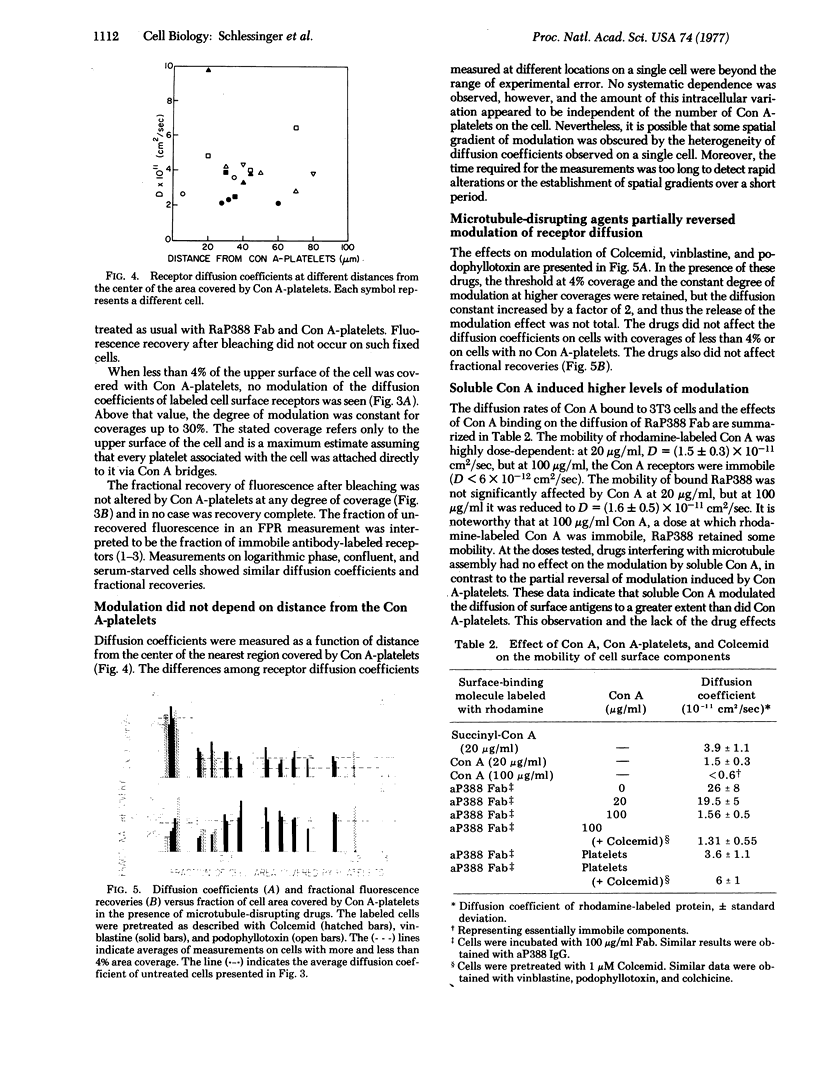
In order to test the anchorage modulation hypothesis, the fluorescence photobleaching recovery method was used to measure the global inhibition of cell surface receptor mobility induced in 3T3 mouse fibroblasts by local binding of platelets labeled with concanavalin A (Con A). By measuring the diffusion of antibody-labeled cell surface receptors at various points on the cell surface, two states, immobile and mobile, were distinguished in the receptor population. Bound Con A-platelets, occupying between 4% and 30% of the cell surface, decreased the diffusion coefficient of the mobile population by a factor of 6. The magnitude of this effect was independent of distance from the sites of the bound Con A-platelets, demonstrating the propagated and nonlocal properties of the modulation effect. The immobile fraction of the population was not changed by Con A-platelet binding. Modulation of the diffusion constant of mobile receptors was partially reversed by treatment with microtubule-disrupting agents such as Colcemid and Vinca alkaloids. High doses of soluble Con A induced even higher levels of modulation than Con A-platelets, but reversal by microtubule-disrupting drugs was observed. These experiments provide additional support for the anchorage modulation hypothesis and provide a measure of the nature and degree of mobility at the molecular level. They also put important constraints on the hypothesized interactions among submembranous components (microtubules and microfilaments) of surface modulating assemblies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Yahara I., Edelman G. M. Morphology, motility, and surface behavior of lymphocytes bound to nylon fibers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1149–1153. doi: 10.1073/pnas.71.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Sela B. A., Wang J. L., Edelman G. M. Antibodies reactive with cell surface carbohydrates. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1127–1131. doi: 10.1073/pnas.72.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist K. G., Ehrnst A. Cytoskeletal control of surface membrane mobility. Nature. 1976 Nov 18;264(5583):226–231. doi: 10.1038/264226a0. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Electron microscopic analysis of the modulation of lymphocyte receptor mobility. Exp Cell Res. 1975 Mar 1;91(1):125–142. doi: 10.1016/0014-4827(75)90150-0. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Modulation of lymphocyte receptor mobility by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1579–1583. doi: 10.1073/pnas.72.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. The effects of concanavalin A on the mobility of lymphocyte surface receptors. Exp Cell Res. 1973 Sep;81(1):143–155. doi: 10.1016/0014-4827(73)90121-3. [DOI] [PubMed] [Google Scholar]