Abstract
Abiotic stresses cause serious damage to plants; therefore, plants undergo a complicated stress response through signal transduction originating from environmental stimuli. Here we show that a subset of short-chain leaf volatiles with an α, β-unsaturated carbonyl bond in their structure (reactive short-chain leaf volatiles, RSLVs) like (E)-2-hexenal and (E)-2-butenal can act as signal chemicals that strongly induce the gene expression of abiotic-related transcription factors, such as heat stress-related transcription factors (HSFA2, MBF1c) and other abiotic stress-related transcription factors (DREB2A, ZATs). RSLV-induced expression of HSFA2 and MBF1c was eliminated in HSFA1s-, known as heat stress response master regulators, knockout mutant, whereas those of DREB2A and ZATs were not, suggesting that the RSLV signaling pathway is composed of HSFA1-dependent and -independent pathways. RSLV treatment induced production of chaperon proteins, and the RSLV-treated Arabidopsis thus demonstrated enhanced abiotic stress tolerance. Because oxidative stress treatment enhanced RSLV production, we concluded that commonly found RSLVs produced by environmental stresses are powerful inducer of abiotic stress-related gene expression as oxidative stress signals.
As sessile organisms, plants are constantly exposed to widely varying and unfavorable environmental conditions, such as drought and extreme temperature, which are major limiting factors in crop production1. Under environmental stress, disturbance of the metabolic balance in oxidative organelles often results in enhanced production of reactive oxygen species (ROS)2. The chloroplast in stressed conditions is one of the major sites producing ROS; disturbance of the photochemical reaction leads to ROS production, which is enhanced by conditions limiting CO2 fixation such as drought and salt stresses and the combination of these conditions with high light2,3. Plants experiencing environmental stresses exhibit complicated responses to overcome the stresses. In the response process, physical stimuli from the environment are perceived and transduced to biochemical processes, resulting in the induction of a series of abiotic stress-related gene expression. Recently, ROS itself4, ROS-related chemicals such as carotenoid oxidation products5 and lipophilic reactive electrophilic species6 are recognized as important signaling chemicals involved in environmental stress responses in addition to phytohormones such as abscisic acid, salicylic acid, jasmonic acid and ethylene.
Previously, we determined that short-chain α, β-unsaturated carbonyls are generated from peroxidized polyunsaturated fatty acids (PUFAs) such as linolenic acid (C18:3) and cis-3, cis-9, cis-12-hexadecatrienoic acid (C16:3), the richest PUFAs in thylakoid membranes7. Through their highly reactive electrophilic α, β-unsaturated carbonyl bonds, these carbonyls can easily modify proteins in stressed plants7,8. In this study, we name such short-chain volatiles having α, β-unsaturated carbonyl bonds in their structure reactive short-chain leaf volatiles (RSLVs) and hypothesize that RSLVs act as signal molecules to induce abiotic gene expression because chemicals with α, β-unsaturated carbonyl bonds have been pointed out to be biologically active9,10.
Results and discussion
RSLVs induced various abiotic-related transcription factors
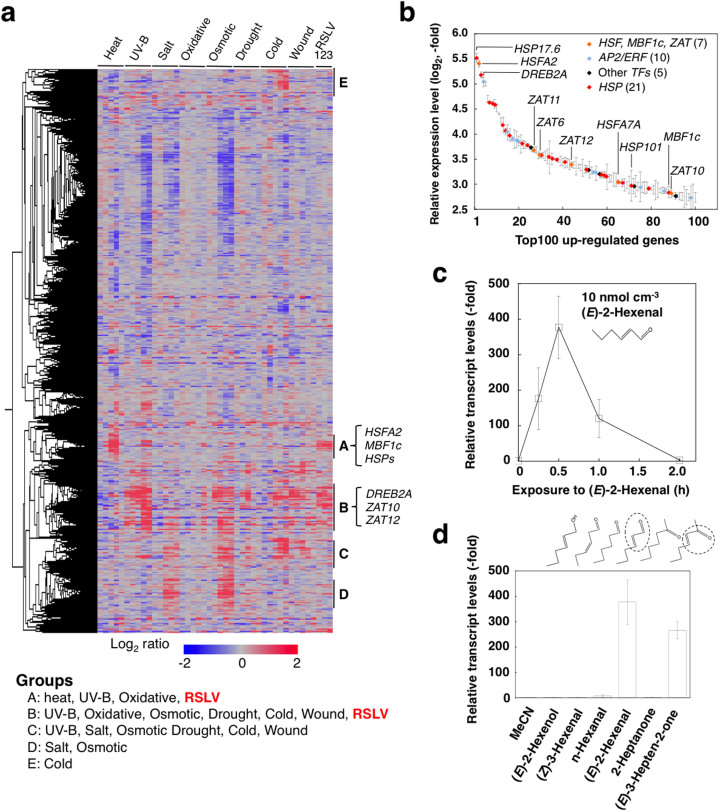
We used (E)-2-hexenal as a model RSLV because (E)-2-hexenal is a widely distributed C6 RSLV known as a green leaf volatile (GLV). An overview of the whole gene expression pattern obtained by a comprehensive microarray analysis showed that vaporized (E)-2-hexenal treatment (10 nmol cm−3 for 30 min, photograph is shown in Supplementary Fig. S1a) induced genes up-regulated in various abiotic stress responses (Fig. 1a, lane RSLV1). Comparing gene expression patterns under various abiotic stresses allowed us to classify up-regulated genes into the following 5 groups (Fig. 1a): heat, UV-B and oxidative stresses-responsive genes (Group A); various stresses-responsive genes (Group B); genes that respond to salt, oxidative, osmotic drought, cold and wounding (Group C); salt- and osmotic-responsive genes (Group D); and cold-responsive genes (Group E). Among these groups, gene expression of Groups A and B were stimulated by (E)-2-hexenal treatment. Many of the 100 most highly up-regulated genes were abiotic stress-related genes (Fig. 1b, Supplementary Table S1) as follows: genes encoding HSPs (21 genes); heat or oxidative stress-related transcription factors (7 genes), including HSFA211, MBF1c12 and ZATs13; abiotic-related AP2/ERF transcription factors (10 genes), including DREB2A14; and other transcription factors (5 genes).
Figure 1. Abiotic stress-related genes were up-regulated by RSLV treatment in Arabidopsis.
(a), Heat map constructed with the whole gene expression. Expression data used are data from shoots collected over the course (0.25, 0.5, 1, 3, 6 h of heat, UV-B, drought or wound stresses) and (0.5, 1, 3, 6, 12 h of oxidative, salt, osmotic or cold stresses) obtained from the AtGenExpress database. RSLVs used for obtaining expression data are (E)-2-hexenal (lane 1), (E)-2-butenal (lane 2) and 3-hepten-2-one (lane 3). Genes induced by heat and various stresses induced were classified into Groups A and B, respectively. In addition, responsive genes to salt, oxidative, osmotic drought, cold and wound (Group C), salt- and osmotic-responsive genes (Group D) and cold-responsive genes (Group E) are shown. (b), Transcription factors and HSPs in the 100 most highly up-regulated genes. Expression was induced by (E)-2-hexenal treatment. Symbols indicate the HSF and ZAT genes (orange), AP2/ERF genes (blue), other transcription factor genes (black), and HSP genes (red). Symbols of other genes are omitted. Data are means ± SE (n = 3). Detailed list is shown in Table S1. (c) and (d), Arabidopsis plants were exposed to (E)-2-hexenal (10 nmol cm−3) for various time periods (0 to 2 h) (c), or series of C6 GLVs and their analogues (each 10 nmol cm−3) for 30 min (d).
RSLVs strongly and rapidly induced HSFA2 gene expression
Because HSFA2 and HSPs were prominently induced by (E)-2-hexenal treatment, detailed biological activity of (E)-2-hexenal was examined using the heat shock factor (HSF)-heat shock protein (HSP) system. Vaporized (E)-2-hexenal rapidly and powerfully induced HSFA2 expression within 30 min (Fig. 1c). This expression was transient and terminated after 2 h. We confirmed that these treatments increased the internal (E)-2-hexenal concentration comparable to the intracellular concentration of intact higher plants15,16. At 10 min after applying (E)-2-hexenal at 10 nmol cm−3 to Arabidopsis, the internal concentration of (E)-2-hexenal exhibited a transient increase of up to 25 nmol g−1 fresh weight (FW) (supplementary Fig. S1b, c). We tested the effects of other GLVs on HSFA2 expression: (E)-2-hexenol, (Z)-3-hexenal, and n-hexanal did not induce HSFA2 expression (Fig. 1d). Among ketones, (E)-3-hepten-2-one but not 2-heptanone induced HSFA2 expression, suggesting that the α, β-unsaturated carbonyl bond moiety was essential for HSFA2 induction. Induction of HSFA2 was dose-dependent above 2.5 nmol cm−3 and saturated at 25 nmol cm−3 (Supplementary Fig. 2).
RSLVs having longer hydrocarbon chains act as signal molecules with less cytotoxic effect
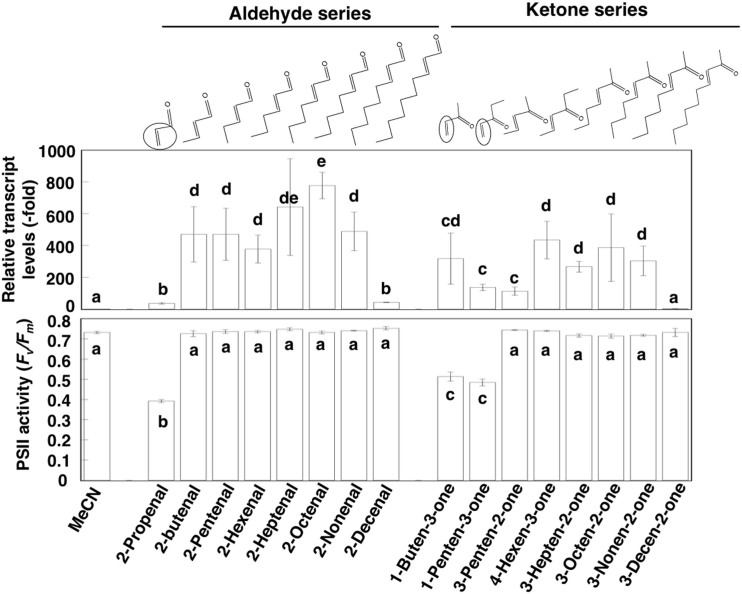
To determine the effect of carbon chain length on HSFA2 induction, we compared the effects of a series of RSLVs with various carbon chain lengths, and found that RSLVs with chain lengths of C4 to C9 were effective with a slightly higher induction in the aldehyde form than in the ketone form (Fig. 2). Expression patterns obtained by microarray analyses using (E)-2-butenal- or (E)-3-hepten-2-one-treated Arabidopsis, were essentially homologous to that of (E)-2-hexenal (Fig. 1a, lane RSLV2 and 3), suggesting that the RSLVs exhibit identical induction activity against abiotic stress-related genes. However, a member of RSLVs is potentially photosynthesis damaging agents, as shown by our previous in vitro study8. The ratio Fv/Fm, the maximum photochemical quantum efficiency of photosystem II (PSII), is used as a measure of stress response because PSII is one of the sites most sensitive to α, β-unsaturated carbonyls8,10, thus we examined the photosynthesis damaging activity of RSLVs by measuring Fv/Fm in Arabidopsis at 23°C. As a result, except vinyl group (H2C = CH-) containing RSLVs such as 2-propenal, 1-buten-3-one, and 1-penten-3-one, RSLVs did not damage PSII (Fig. 2, lower panel, Supplementary Fig. S3). Therefore, the RSLVs with chain lengths of C4 to C9 having no vinyl group possibly act as signal molecules with less cytotoxic effect.
Figure 2. Effect of chain-length of RSLVs on Induction of HSFA2 expression and PSII activity.
Arabidopsis plants were exposed to (E)-2-hexenal (10 nmol cm−3) a series of RSLVs of aldehyde form (each 10 nmol cm−3) for 30 min, or a series of RSLVs of ketone form (each 10 nmol cm−3) for 30 min (upper panel). Expression of the HSFA2 gene was determined using qRT-PCR. Relative transcript levels were normalized to ACTIN2 mRNA. The expression level of the 0 h exposure sample was set to 1. Effect of RSLVs on PSII activity was determined by treatment of the indicated α, β-unsaturated aldehydes or ketones (each 25 nmol cm−3) for 90 min to Arabidopsis plants, and then PSII activity was measured (lower panel). The chemical structures of the RSLVs are shown above the graphs. Dotted and solid circles indicate the α, β-unsaturated carbonyl bond moieties and vinyl group, respectively. Data are means ± SE (n = 3 in upper panel, n = 5 in lower panel). Values followed by the same letter are not significantly different according to Tukey-Kramer (P < 0.05).
Evaluation of endogenous RSLVs on HSFA2 expression
Production of (E)-2-hexenal via an enzymatic pathway (Supplementary Fig. S4a) is assumed to be activated when plant tissues are disintegrated by physical factors, such as pest invasion, wounding or freezing17. In Arabidopsis ecotype No-0, which can produce (E)-2-hexenal enzymatically, the (E)-2-hexenal content in leaves reached 110 nmol g−1 FW when the leaf was disrupted16. However, in Arabidopsis ecotype Col-0 used in this study, (E)-2-hexenal is undetectable because hydroperoxide lyase, which is necessary for producing C6 GLVs, is nonfunctional truncated protein due to 10-nucleotide deletion in its first exon18. This difference allowed us to determine which RSLVs were produced by non-enzymatic peroxidation of PUFAs. Oxidative stress caused by treatment with 10 μM methylviologen (MV) under illumination resulted in an increase of (E)-2-butenal (Supplementary Fig. S5a). Full-scan spectra of fragment ions (Supplementary Fig. S5b) and selected reaction monitoring using major fragment ions (Supplementary Fig. S5c) confirmed the identity of this endogenous compound. Concomitant with increase in HSFA2 mRNA expression (Supplementary Fig. S6a, column Col-0), these results suggest that (E)-2-butenal produced by non-enzymatic peroxidation of PUFAs (possible pathway is shown in Supplementary Fig. S4b) can act as signaling chemicals that induce HSFA2 expression.
To assess the effect of endogenous RSLVs on HSFA2 expression, we analyzed HSFA2 mRNA expression in aor mutants, which are deficient in chloroplastic alkenal/one oxidoreductase (AOR)19, the enzyme that catalyzes the saturation of α, β-unsaturated carbonyl bonds in reactive carbonyls such as RSLVs (Supplementary Fig. S6e). Previously, we found that aor exhibited high sensitivity to MV treatment concomitant with accumulation of reactive carbonyls including RSLVs20. In this study, both aor and Col-0 showed similar levels of (E)-2-butenal and HSFA2 expression under normal condition, and MV treatment enhanced accumulation of (E)-2-butenal and HSFA2 expression in aor and Col-0 (Supplementary Fig. S6a, b). The enhancement of HSFA2 expression in the MV-treated aor was higher than that in MV-treated Col-0, corresponding to higher (E)-2-butenal accumulation in aor (Supplementary Fig. S6a, b). This result indicates that total of RSLVs including (E)-2-butenal and minor RSLVs whose concentration is lower than detection-limit of our analysis might induce HSFA2 higher in aor mutant than Col-0. Similar results were obtained from ultraviolet B (UV-B) stress treatment (Supplementary Fig. S6c, d) that accompanies ROS production21 and lipid peroxidation22. These enhancements of HSFA2 expression by stress treatments support the hypothesis that RSLVs are involved in the induction of HSFA2 expression in vivo.
RSLV signaling is transmitted via both HSFA1-dependent and -independent pathways
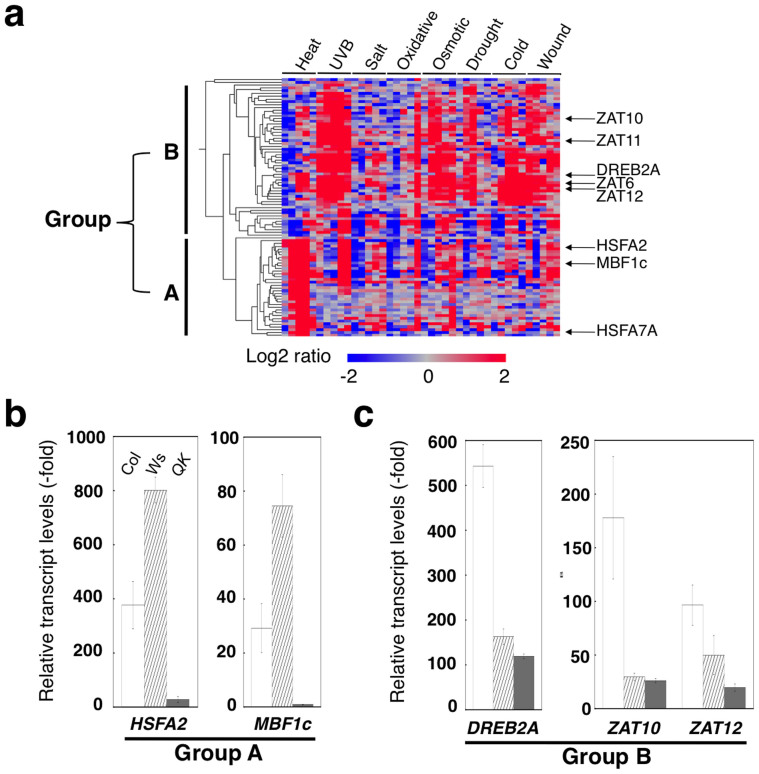
In a heat-stress response, HSFA1s act as master regulators to drive the HSF-HSP system23. To determine whether RSLV stimulates HSFA1-mediated HSP expression, we used an HSFA1a/1b/1d/1e quadruple knockout mutant (QK), which exhibited no HSFA2 expression when exposed to heat conditions23. In the genomic background ecotypes of QK, wild-type Col-0 and Ws-0, HSFA2 and MBF1c (belonging to group A, Fig. 3a), and DREB2A, ZAT10 and ZAT12 (belonging to group B, Fig. 3a) were induced by (E)-2-hexenal treatment (Fig. 3b, c). In (E)-2-hexenal–treated QK, HSFA2 and MBF1c expression was almost eliminated (Fig. 3b), whereas expression of DREB2A and ZAT10 remained at a similar level to that of Ws-0 (Fig. 3c). These results suggested that RSLV-mediated gene expression involves heterogeneous pathways, i.e. the HSFA1-dependent and HSFA1-independent pathways.
Figure 3. RSLV-induced genes are divided into HSFA1-dependent or –independent groups.
(a), Heat map constructed with the 100 most highly up-regulated genes by (E)-2-hexenal treatment. (b), Expression of transcription factors in (E)-2-hexenal–treated Col-0 (Col, open column), Ws-0 (Ws, shaded column) and HSFA1 quadruple knockout mutant (QK, gray column). A and B mean groups classified in panel (a).
Tomato HSFA2s, and rice HSFA2s and DREB2A were also induced by (E)-2-hexenal
(E)-2-Hexenal is a common RSLV that is also detected in tomato24 and rice25, thus might act as a signal chemical among various plant species. To explore this possibility, we examined the induction of HSFs by (E)-2-hexenal in a model tomato cultivar (Solanum lycopersicum ‘Micro-Tom') and rice (Oryza sativa ‘Nipponbare') because these species in which the heat-stress response mechanism has been well-characterized. The results of tomato were similar to those described for Arabidopsis in that the tomato HSFA2 genes were induced by (E)-2-hexenal. The induction profiles of other classes of HSFs—HSFA1 and HSFB1 in tomato were similar to those in Arabidopsis (Supplementary Fig. S7a, b). In the case of a monocotyledonous plant rice, (E)-2-hexenal also up-regulated heat-inducible OsHSFA2s and abiotic stress-inducible OsDREB2A (Supplementary Fig. S7c). These similarities of gene expression profiles among species indicate that RSLVs might be common signal chemicals.
RSLV treatment could enhance abiotic stress tolerance
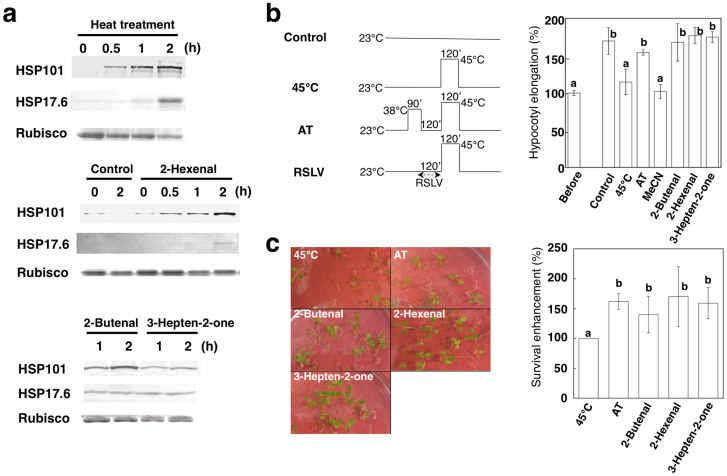
Because RSLVs induced HSF and HSP gene expression, protein expression enhancement by RSLV treatment was confirmed by detecting two HSPs: HSP101 (encoded by At1g74310) and HSP17.6 (encoded by At1g53540). As shown in Fig. 4a, HSP101 and HSP17.6 were induced by (E)-2-hexenal, (E)-2-butenal and (E)-3-hepten-2-one treatment at 23°C within 2 h, whereas the levels in acetonitrile (MeCN)-treated control plants remained low. Finally, we investigated an effect of RSLV treatment on abiotic stress tolerance. RSLV-induced thermotolerance was assessed by evaluating hypocotyl elongation26 and survival tests, because RSLV treatment could enhance HSFA2 expression and HSP17.6 production (Supplementary Fig. S8) those confer acquired thermotolerance27. After 2.5 days of growth on vertical plates in the dark, the seedlings were applied to the hypocotyl elongation test (Fig. 4b). After heat treatment at 45°C for 2 h, the control and solvent-control seedlings stopped developing, whereas seedlings pretreated at 38°C for 90 min (for gaining acquired thermotolerance) or RSLV-treated seedlings at 23°C for 120 min continued hypocotyl elongation. The thermotolerance enhancing effect of (E)-2-hexenal was not observed in HSFA1s-deficient QK mutants (Supplementary Fig. S9a), indicating that the physiological importance of HSFA1-dependent pathway in heat stress response. In the survival enhancement test, RSLV treatments enhanced thermotolerance similar level to that of acquired thermotolerance (Fig. 4c). In addition, RSLV treatments could enhance protection of PSII from heat- or UV-B-derived damages (Supplementary Fig. S9b, c).
Figure 4. Biological effects of RSLV treatment of Arabidopsis.
(a), After the indicated RSLV treatment or heat treatment at 38°C, expression of HSP101 and HSP17.6 proteins was detected by western blot analysis. Rubisco was stained using Coomassie Brilliant Blue R-250 as a loading control. These images are cropped from original images shown in Supplementary Fig. S11. (b), The 2.5-day-old dark-grown seedlings (Before) were pretreated at 38°C for 90 min to acquire thermotolerance (AT) or 10 μM indicated RSLVs or solvent control (MeCN) for 2 h and then heat-stressed at 45°C for 2 h. Seedling were returned to 23°C in the dark and length was measured after 2.5 days. Length of seedlings before treatment was set to 100%, and elongation of each treatment was calculated. Schemes of treatment are shown above the graph. (c), Survival enhancement was calculated by survival rate determined on 3 days after treatments with same scheme as shown in panel (b). Left; Representative photographs of Arabidopsis plants on 7 days after heat stress treatments. Right; Survival enhancement by RSLV treatment calculated from 3 independent assays. Survival rate of 45°C sample was set to 100%. Values followed by the same letter are not significantly different according to Tukey-Kramer (n = 4 or 5; P < 0.05).
Possible role of RSLVs in abiotic stress responses
RSLVs have been widely detected among plant species, and increased RSLV production has been observed under abiotic stresses6 including heat stress (Fig. S10). Production of (E)-2-hexenal was detected in a photoinhibition-sensitive Arabidopsis mutant (Col-0 background) npq1 by intense light conditions28, which could cause ROS to be overproduced from the loss of energy dissipation. Additionally, in tomato plants, production of (E)-2-hexenal was enhanced under heat and cold stresses29. Furthermore, increased levels of (E)-2-pentenal and (E)-2-hexenal were also detected in tobacco plants under photooxidative stress condition15. Consequently, non-enzymatic pathway-derived small 2-alkenals, and both enzymatic and non-enzymatic pathways-derived (E)-2-hexenal can act as endogenous signal chemicals that respond to abiotic stresses.
Volatiles such as isoprenoids play important roles in various stresses tolerance30. Also in the case of RSLVs, our results indicate that RSLVs stimulate heterogeneous signal transduction in response to abiotic stress (Fig. 5). One signal transduction pathway is an HSFA1-dependent pathway expressing proteotoxic stress-related genes that contribute to HSP production to maintain protein homeostasis. The other signal transduction pathway is mediated by HSFA1-independent pathway expressing various abiotic stress-response genes. RSLVs stimulate both pathways as oxidative stress signals to induce proteotoxic and abiotic stress-response genes.
Figure 5. Hypothesized RSLVs-related signaling pathway in abiotic stress accompanied by oxidative stress.
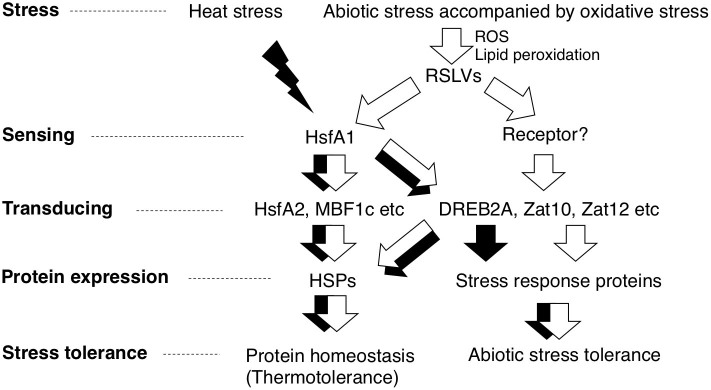
RSLV stimulates both HSFA1-dependent and –independent pathways (white arrows). RSLVs are possibly involved in the upstream of signaling pathway triggered by abiotic stresses accompanied by oxidative stress. Proteotoxic stress such as heat and ROS are accepted by HSFA1s to drive HSFA1-denpendent pathway (black arrows).
Methods
Chemicals
2-Propenal, (E)-2-butenal, (E)-2-pentenal, (E)-2-heptenal, (E)-2-octenal, (E)-2-nonenal, (E)-2-decenal, (E)-3-hepten-2-one, (E)-3-octen-2-one, (E)-3-nonen-2-one, (E)-3-decen-2-one, (E)-2-hexenol, 2-hexanal, and 2-heptanone were purchased from Tokyo Chemical Industry (Tokyo, Japan). 1-Penten-3-one and 3-penten-2-one were purchased from Sigma-Aldrich (St. Louis, MO, USA). (Z)-3-Hexenal was obtained from Bedoukian Research Inc. (Danbury, CT, USA). (E)-2-Hexenal, (E, Z)-4-hexen-3-one, and other reagents were purchased from Wako Pure Chemicals (Osaka, Japan).
Plant materials
Seeds of Arabidopsis thaliana (ecotype Columbia-0: Col-0; Wassilewskija: Ws-0) and tomato (Solanum lycopersicum ‘Micro-Tom') were sown on Jiffy-7 peat pellets (Sakata Seed Co., Yokohama, Japan) and kept at 4°C for 3 days in the dark. Then plants were transferred to the conditions of a 14-h–light (80 μmol photons m−2 s−1)/10-h–dark cycle at 23°C. The AOR-deficient Arabidopsis mutant aor, which was previously identified as a T-DNA knockout line of AOR16, was obtained from the Arabidopsis Biological Resource Center (ABRC, Columbus, OH, USA). HSFA1s quadruple knock-out mutant (QK) was generously gifted by Dr Y.-Y. Charng, National Taiwan University, Taiwan. Seeds of rice (Oryza sativa L., cv. Nipponbare) were immersed in water for a day at 4°C, and the transferred to the conditions of a 14-h–light (80 μmol photons m−2 s−1)/10-h–dark cycle at 25°C. Oxidative treatment was performed by immersing the aerial parts of plants in 10 μM methylviologen (MP Biomedicals, Solon, OH, USA) under illumination (80 μmol photons m−2 s−1). UV-B treatment was performed by irradiation of UV light (VL-6MC, 312 nm tube, Vilber Lourmat, France) with 1 mW cm−2. Heat treatment was performed by exposing at 40°C in the presence of light (80 μmol photons m−2 s−1).
Volatile treatment
Plants were placed in a transparent plastic box (340 cm3, Nippon Genetics, Tokyo, Japan). Volatiles were diluted with MeCN, which does not induce HSFA2 mRNA. Each volatile tested (total volume of 3 μl) was absorbed into a piece of paper towel attached to the inside of the cover. The cover was immediately set on the box (Supplementary Fig. S1a), and the plants were incubated at 25°C under illumination (80 μmol photons m−2 s−1). MeCN-treated plants were used as controls.
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was purified by using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany), and then cDNA was synthesized by using a RevaTra Ace kit (Toyobo, Osaka, Japan). Gene-specific primers were chosen with the use of the Primer3 program (http://frodo.wi.mit.edu/). Primer sequences are shown in Table S2. Quantitative real-time RT-PCR (qRT-PCR) was performed with the use of Thunderbird SYBR Green qPCR Mix (Toyobo) and a LineGene Real-time PCR Detection System (FQD-33A, BioFlux, Tokyo, Japan). For analysis of relative transcript levels, ACTIN2 mRNA was used as an internal standard in all qRT-PCR experiments; the expression levels of genes of interest were normalized to that of ACTIN2 by subtracting the cycle threshold (CT) value of ACTIN2 from the CT value of the gene of interest. The expression level of MeCN-treated controls was set to 1. For determination of absolute copy number of HSFA2, we used samples including equal amount of ACTIN2 determined by qRT-PCR and the pMD20 plasmid (Takara Bio Inc., Otsu, Japan) containing the HSFA2 (3.6 kbp, 254 copies/fg) as a reference matrix.
Microarray RNA sample preparation and hybridizations
Total RNA was purified by using an RNeasy Plant Mini Kit (Qiagen) from at least 6 plants. The double-strand (ds) cDNA was generated with a modified procedure of the Superscript Choice System (Life Technologies, Carlsbad, CA). Briefly, the 1st strand cDNA was synthesized from 10.0 μg total RNA by 1.0 unit SuperScript II reverse transcriptase (Life Technologies) in the presence of 100 pmoles Oligo dT(20) primer. After 2nd strand synthesis, the template RNA was digested with RNase A, then the synthesized DNA was purified with phenol:chloroform:isoamyl alcohol. The purified DNA was precipitated in ethanol, and the pellet was washed, dried, reconstituted and quantified. cDNA samples were labeled using the random priming method with Cy3-labeled random nonamer as primers and Klenow DNA polymerase at 37°C for 2 h (NimbleGen One-color Labeling Kit, NimbleGen Roche, Madison, WI). The labeled DNA was precipitated in isopropanol, and the pellet was washed, dried, reconstituted and quantified. For each hybridizations, 4 μg of labeled DNA was diluted by NimbleGen sample tracking control kit buffers respectively, and be added with NimbleGen hybridization buffers according to the manufacturer's protocols. The arrays (ATH6_60mer_expr X4) were hybridized with labeled DNA on a NimbleGen Hybridization System at 42°C for 16 h. Arrays were washed by NimbleGen wash buffer kit according to the manufacturer's protocols and scanned using an Axon GenePix 4000B scanner at 5 μm resolution. The microarray experiments using the Agilent Arabidopsis ver4.0 (44 k) microarray (Agilent Technologies) with a one-color method were performed according to the manufacturer's instructions. Data extraction, normalization and production of heat map by UPGMA clustering were performed by Subio Platform software (Subio, Kagoshima, Japan), and Microsoft Excel was used to organize and interpret the data. “Ratio” values are the mean of 3 independent experiments, and genes showing a value above 2.0 or below 0.5 (99.8% confidence) were considered as up- or down-regulated, respectively. Datasets of gene expression under each stressed condition were obtained from AtGenExpress database (The Arabidopsis Information Resource, The Ohio State University, OH, USA). Expression data used in this figure are data of shoot in time course (0.25, 0.5, 1, 3, 6 h of heat, UV-B, drought or wound stresses) and (0.5, 1, 3, 6, 12 h of oxidative, salt, osmotic or cold stresses).
Identification of RSLVs
Harvested plants were immersed in MeCN (2 ml), and 1 μl of 10 mM 2-ethylhexanal was added as an internal standard. After incubation for 30 min at 60°C, 1 ml of the solution was transferred to another glass tube, and 38 μl of HCOOH and 50 μl of 20 mM dinitrophenyl hydrazine dissolved in MeCN were added. After incubation for 60 min at room temperature, 1 ml of saturated NaCl solution and 0.1 g of NaHCO3 were added and well mixed. After incubation for 20 min, 0.5 ml of the MeCN layer was transferred to a plastic tube and evaporated. The resultant residue was dissolved in 200 μl of MeCN, and the solution was filtered through a Cosmonice filter (pore size 0.45 μm, Nacalai Tesque, Kyoto, Japan). The resultant cleared solution was used as the DNP-carbonyl preparation for HPLC analysis. HPLC analysis was performed by the method described previously20,31,32. Data were analyzed by using PowerChrom software (eDAQ Pty Ltd., Denistone East, NSW, Australia).
For accurate identification of DNP-RSLVs, DNP-RSLVs were subsequently analyzed by liquid chromatography-mass spectrometry (LC/MS/MS, Acquity UPLC/TQD; Waters, Milford, MA, USA) using YMC-PACK C4 (φ2.0 × 100 mm, 3 μm, Waters). For reverse phase chromatography of RSLV-DNPs, the elution of the samples was carried out with 10% tetrahydrofuran (solvent A2) and acetonitrile (solvent B2), and the mobile phase was changed from 35% (v/v) B2–100% (vol/vol) at 2 and 21.5 min after the injection, respectively, at a flow rate of 0.3 mL·min−1. Elution was monitored by Photodiode and MS analysis with ES-negative mode. The column temperature was 40°C. MS/MS analysis conditions were as follows: Declustering potential, 40; collision energy, 40 V; and parent ion (m/z), 249 for (E)-2-butenal-DNP.
Protein analysis
Protein was extracted with 5 volumes of 50 mM HEPES-NaOH, pH 7.0. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% (HSP101) or 12% polyacrylamide gels (HSP17.6). The proteins were electroblotted onto polyvinylidene difluoride (PVDF) membranes (ATTO, Tokyo, Japan) according to the manufacturer's instructions, and anti-HSP101 and anti-HSP17.6 antibodies (Agrisera, Vännäs, Sweden) were used for immunochemical detection. Alkaline phosphatase–conjugated secondary antibody was used for visualizing signals with 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium as substrates. The bands were quantified by densitometric analysis using ImageJ software after scanning the blotted membrane.
Measurement of chlorophyll fluorescence
The maximum quantum yield of PSII was estimated from chlorophyll fluorescence measurements by pulse-amplitude–modulated (PAM) fluorometer (Junior-PAM; Walz, Effeltrich, Germany). Plants were dark-adapted at room temperature for 5 min before measuring. The yield of PSII was calculated as the ratio of Fv/Fm.
Thermotolerance test
For a hypocotyl elongation test33, seeds planted on MS plates were wrapped in foil and incubated at 4°C for 3 day, then at 23°C for 2.5 days (Col-0) or 5 days (QK). After length of cotyledons was measured, they were separately treated as follows. i) For 45°C treatment, seedlings were incubated at 45°C for 2 h. ii) For acquired thermotolerance treatment, seedlings were incubated at 38°C for 90 min followed by 2 h at 23°C and then 2 h at 45°C. ii) For volatile treatment, seedlings were treated with 10 nmol cm−3 of (E)-2-butenal, (E)-hexenal or 3-hepten-2-one for 2 h at 23°C and then 2 h at 45°C. After treatment, seedlings were incubated an additional 2.5 days in the dark, then length of seedlings was measured.
For survival test, seeds (at least 30 plants) planted on MS plates were wrapped in foil and incubated at 4°C for 3 day, then at 23°C for 2.5 days. After the seedlings were subjected to the same treatments as elongation test, seedlings were additionally incubated under standard conditions of a 14-h–light (80 μmol photons m−2 s−1)/10-h–dark cycle at 23°C. The percentage of survival plants was calculated by counting the continuously developing plants per total plants after 3 days. Survival enhancement was determined by calculation using survival rate of RSLV treatment sample against that of 45°C sample.
Statistical analysis
Data were analyzed by using the programs of Statistical analysis program (StatPlus, AnalystSoft). Data were subject to ANOVA with post hoc analysis, and means were compared by Tukey-Kramer test (P < 0.05).
Gene identifier
Arabidopsis; Actin2, At3g18780; HSFA2, At2g26150; HSFA1a, At4g17750; HSFA1b, At5g16820; HSFA1d, At1g32330; HSFA1e, At3g02990; HSFB1, At4g36990; DREB2A, At5g05410; ZAT10, At1g27730; ZAT12, At5g59820; MBF1c, At3g24500.
Tomato: Actin, Sl11g005330; HSFA1, Sl08g005170; HSFA2, Sl08g062960; HSFB1, Sl02g090820.
Rice: Actin1, Os03g0718100; HSFA2c, Os10g0419300; HSFA2d, Os03g0161900; HSFA2e, Os03g0795900; DREB2A, Os01g0165000.
Author Contributions
Y.Y. designed the project, performed experiments and wrote the manuscript; M.K. performed experiments using Arabidopsis seedlings. M.M. supervised molecular biological and biochemical analyses. Y.S. supervised chemical analysis.
Supplementary Material
Supplementary Figures and Tables
Acknowledgments
Dr Y.-Y. Charng, National Taiwan University, Taiwan, generously gifted HSFA1s quadruple knock-out mutant. This study was supported by a Grant-in-aid for Exploratory Research (Y.Y., 21658112) and a Grant-in-Aid for Scientific Research (Y.Y., 23580456) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a Grant from Phytochrome Inc., Tokyo, Japan.
Accession codes: The transcriptome expression data from RSLV-treated Arabidopsis seedlings have been deposited under the following accession codes: GSM1569068-1569075.
References
- Boyer J. S. Plant productivity and environment. Science 218, 443–448 (1984). [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7, 405–410 (2002). [DOI] [PubMed] [Google Scholar]
- Foyer C. H. & Noctor G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 119, 355–364 (2003). [Google Scholar]
- Miller G., Shulaev V. & Mittler R. Reactive oxygen signaling and abiotic stress. Physiol. Plant 133, 481–489 (2008). [DOI] [PubMed] [Google Scholar]
- Ramel F. et al. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 109, 5535–5540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M. J. & Berger S. Reactive electrophilic oxylipins: Pattern recognition and signalling. Phytochemistry 70, 1511–1521 (2009). [DOI] [PubMed] [Google Scholar]
- Yamauchi Y. et al. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 46, 786–793 (2008). [DOI] [PubMed] [Google Scholar]
- Yamauchi Y. & Sugimoto Y. Effect of protein modification by malondialdehyde on the interaction between oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 231, 1077–1088 (2010). [DOI] [PubMed] [Google Scholar]
- Vollenweider S., Weber H., Stolz S., Chételat A. & Farmer E. E. Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 24, 467–476 (2000). [DOI] [PubMed] [Google Scholar]
- Alméras E. et al. Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34, 205–216 (2003). [DOI] [PubMed] [Google Scholar]
- Nishizawa A. et al. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 48, 535–547 (2006). [DOI] [PubMed] [Google Scholar]
- Suzuki N. et al. Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiol. 139, 1313–1322 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiełbowicz-Matuk A. Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Sci. 185–186, 78–85 (2012). [DOI] [PubMed] [Google Scholar]
- Mizoi J., Shinozaki K. & Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 1819, 86–96 (2012). [DOI] [PubMed] [Google Scholar]
- Mano J., Tokushige K., Mizoguchi H., Fujii H. & Khorobrykh S. Accumulation of lipid peroxide-derived, toxic α, β-unsaturated aldehydes (E)-2-pentenal, acrolein and (E)-2-hexenal in leaves under photoinhibitory illumination. Plant Biotech. 27, 193–197 (2010). [Google Scholar]
- Matsui K., Sugimoto K., Mano J., Ozawa R. & Takabayashi J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 7, e36433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9, 274–280 (2006). [DOI] [PubMed] [Google Scholar]
- Duan H., Huang M. Y., Palacio K. & Schuler M. A. Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiol. 139, 1529–1544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y., Hasegawa A., Taninaka A., Mizutani M. & Sugimoto Y. NADPH-dependent reductases involved in the detoxification of reactive carbonyls in plants. J. Biol. Chem. 286, 6999–7009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y., Hasegawa A., Mizutani M. & Sugimoto Y. Chroloplastic NADPH-dependent alkenal/one oxidoreductase contributes to the detoxification of reactive carbonyls produced under oxidative stress. FEBS Lett. 586, 1208–1213 (2012). [DOI] [PubMed] [Google Scholar]
- AH-Mackerness S., John C. F., Jordan B. & Thomas B. Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett. 489, 237–242 (2001). [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Fukumoto R., Kasahara H., Sakaki T. & Kitao M. Peroxidation of lipids and growth inhibition induced by UV-B irradiation. Plant Cell Rep. 14, 566–570 (1995). [DOI] [PubMed] [Google Scholar]
- Liu H. C., Liao H. T. & Charng Y. Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 34, 738–751 (2011). [DOI] [PubMed] [Google Scholar]
- Buttery R. G., Ling L. C. & Light D. M. Tomato leaf volatile aroma components. J. Agric. Food Chem. 35, 1039–1042 (1987). [Google Scholar]
- Hernandez H. P., Hsieh T. C. Y., Smith C. M. & Fischer N. H. Foliage volatiles of two rice cultivars. Phytochemistry 28, 2959–2962 (1989). [Google Scholar]
- Hong S. W. & Vierling E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc. Natl. Acad. Sci. USA 97, 4392–4397 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafny-Yelin M., Tzfira T., Vainstein A. & Adam Z. Non-redundant functions of sHSP-CIs in acquired thermotolerance and their role in early seed development in Arabidopsis. Plant Mol. Biol. 67, 363–373 (2008). [DOI] [PubMed] [Google Scholar]
- Loreto F., Barta C., Brilli F. & Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 29, 1820–1828 (2006). [DOI] [PubMed] [Google Scholar]
- Copolovici L., Kännaste A., Pazouki L. & Niinemets U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 169, 664–672 (2012). [DOI] [PubMed] [Google Scholar]
- Vickers C. E., Gershenzon J., Lerdau M. T. & Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chem. Biol. 5, 283–291 (2009). [DOI] [PubMed] [Google Scholar]
- Matsui K., Sugimoto K., Kakumyan P., Khorobrykh S. A. & Mano J. Volatile oxylipins and related compounds formed under stress in plants, Methods in Molecular Biology ‘Lipidomics' Vol. 580 (Armstrong, D. ed.) 17–28 (Humana Press, Totowa, NJ, 2009). [DOI] [PubMed] [Google Scholar]
- Yin L., Mano J., Wang S., Tsuji W. & Tanaka K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 152, 1406–1417 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. W. & Vierling E. Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc. Natl. Acad. Sci. USA 97, 4392–4397 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Tables