Abstract
The aryl hydrocarbon receptor (AhR) mediates the control of environmental toxicity, and modulates the development and pathogenesis of the cardiovascular system. However, little is known about the role of AhR in coronary arterial disease (CAD) susceptibility. We therefore conducted a case-control study in a Chinese population, and assessed the potential association between AhR variants and CAD susceptibility. Compared with the controls, circulating AhR expression was found to be significantly increased in patients with CAD and its subtypes including ST-segment and non-ST-segment elevation myocardial infarction, and stable and unstable angina pectoris. Receiver operating characteristic (ROC) analysis to evaluate the effect of AhR on CAD progression showed it to be a potent biomarker for CAD. Genotype frequencies of AhR rs2066853 differed significantly between CAD and control subjects, while smoking and hyperlipidemia markedly promoted CAD risk relative to the AhR polymorphism. Moreover, a significant difference in AhR variant distribution was observed between the four CAD subtypes with different severities. The expression level and functional polymorphisms of circulating AhR may affect the susceptibility and progression of CAD in Chinese populations. This provides a novel view of the etiology and epidemiology of CAD, and will contribute to the diagnosis and therapy of this severe disease.
The aryl hydrocarbon receptor (AhR) is a transcription factor belonging to the basic helix-loop-helix Per-Arnt-Sim family of DNA-binding proteins. It is often defined as a regulator that mediates the induction of metabolic enzymes and controls the toxicity of environmental compounds1,2. After activation by its ligands, including halogenated aromatic hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons like tobacco procarcinogens, cytoplasmic AhR translocates into the nucleus, dimerizes with the aryl hydrocarbon receptor nuclear translocator (also known as hypoxia-inducible factor-1β), and then transactivates the downstream signaling pathways related to cellular toxicology through binding the target genes3. Thus, AhR is considered to be a sensor of the organisms for environmental stimulus, and enables adaptive responses to be made to the environment by attenuating the potential toxicity of exogenous chemicals.
During the last decade, AhR has also been found to participate in important physiological processes and immune responses4,5. In particular, emerging evidences suggests that it has critical modulatory roles in the development and pathogenesis of the cardiovascular system6. AhR-knockout mice developed cardiac hypertrophy, vascular remodeling, and systemic hypertension7,8,9,10, while AhR activation was found to contribute to the formation and promotion of atherosclerosis through inducing vascular inflammation in apolipoprotein E−/− mice11. These aforementioned pathological results are the main clinical characteristics of coronary arterial disease (CAD), the most common and severe cardiovascular disease, which led us to formulate the hypothesis that AhR could be of pathogenetic importance in CAD. CAD is an inflammatory disease with multifactorial interactions including environmental stimulation and genetic susceptibility. It is well established that smoking, hypertension and atherosclerosis are associated with an increased risk of CAD, but its detailed etiology remain unclear12. An investigation into the potential correlation between AhR polymorphisms and CAD susceptibility would therefore provide a valuable insight into the role of AhR in CAD diagnosis and control.
Human AhR is around 50 kb in size, is located on chromosome 7p15 and contains at least 11 exons and 10 introns13. AhR polymorphisms were previously shown to have negative effects on the sensitivity and affinity of its coding proteins, as well as on activation of the AhR pathway14,15. Among the AhR single nucleotide polymorphisms (SNPs), rs2066853 has attracted considerable attention for its association with the risk of vitiligo, lung cancer, breast cancer and menopausal hot flashes16,17,18,19. However, no association between AhR polymorphisms and CAD has yet been determined. Therefore, based on a case-control study of 939 incident CAD cases and 868 age- and sex frequency-matched CAD-free controls in a Chinese population, we identified AhR as a novel CAD susceptibility gene, and then assessed the potential association between AhR variations (rs2066853) with susceptibility of CAD and its four subtypes20,21.
Results
Clinical characteristics
A total of 939 CAD patients and 868 controls were studied to determine the association of the circulating AhR levels with CAD. The baseline clinical characteristics of all subjects are summarized in Table 1.
Table 1. Baseline characteristics of subjects of CAD and control groups.
| Variables | CAD | Control | P value | P value * |
|---|---|---|---|---|
| Mean age (years) | 64.59 ± 9.99 | 63.59 ± 12.41 | 0.145 | 0.174 |
| Male/famale | 541/252 | 302/159 | 0.324 | 0.324 |
| BMI | 23.13 ± 2.38 | 22.74 ± 2.76 | 0.011 | 0.0165 |
| Smokers, n (%) | 249 (31.3) | 54 (11.7) | <0.001 | <0.001 |
| Hypertension, n (%) | 323 (40.7) | 78 (16.9) | <0.001 | <0.001 |
| Diadetes, n (%) | 234 (29.5) | 41 (8.9) | <0.001 | <0.001 |
| Hyperlipidemia | 363 (45.8) | 114 (24.7) | <0.001 | <0.001 |
| hs-TnI (μg/L) | 1.91 ± 0.58 | 0.012 ± 0.0022 | <0.001 | <0.001 |
| MYO (μg/L) | 140.2 ± 40.46 | 25.69 ± 1.43 | <0.001 | <0.001 |
| CK (μg/L) | 207.5 ± 91.53 | 64.13 ± 3.39 | <0.001 | <0.001 |
| CK-MB (μg/L) | 43.76 ± 4.028 | 17.37 ± 1.17 | <0.001 | <0.001 |
| Glu (mmol/L) | 6.50 ± 6.45 | 5.84 ± 1.97 | 0.008 | 0.0137 |
| CHOL (mg/Dl) | 4.92 ± 1.29 | 4.81 ± 1.22 | 0.158 | 0.172 |
| TG (mmol/L) | 1.65 ± 1.07 | 1.49 ± 1.10 | 0.017 | 0.0227 |
| HDL (mmol/L) | 1.28 ± 0.38 | 1.35 ± 0.41 | 0.006 | 0.012 |
| LDL (mmol/L) | 2.89 ± 1.12 | 2.60 ± 1.13 | <0.001 | <0.001 |
Abbreviations: CAD, coronary arterial disease; BMI, body mass index; Glu, blood sugar; CHOL, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; hs-TnI, high-sensitive troponin I; MYO, myoglobin; CK, creatine kinase; CK-MB, creatine kinase-isoenzyme. Continuous data are expressed as the mean ± SEM. Adjusted for age, sex, smoking, hypertension, diadetes and hyperlipidemia.
*False discovery rate-adjusted P value for multiple hypotheses test using BH method.
There was no significant difference in age or sex between CAD and control groups (P > 0.05), suggesting that the artificial error of clinical sampling was obviated in this study. The mean age of the CAD group was 64.59 years old (±9.99 years), and that of the controls was 63.59 years old (±12.41). The gender ratio (male/female) was 2.15 in the CAD group and 1.90 in the controls. Compared with the control group, some indicators of CAD patients were increased significantly (P < 0.001), including blood sugar (Glu), total cholesterol (CHOL), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). The existing biochemical markers of CAD patients were also significantly higher than in controls, such as high-sensitive troponin I(hs-TnI), myoglobin (MYO), creatine kinase (CK) and CK-MB (P < 0.001), which further confirmed the clinical diagnosis of CAD. Additionally, CAD patients were more inclined to smoke and to have hypertension and hyperlipidemia.
AhR expression in patients with CAD and its subtypes
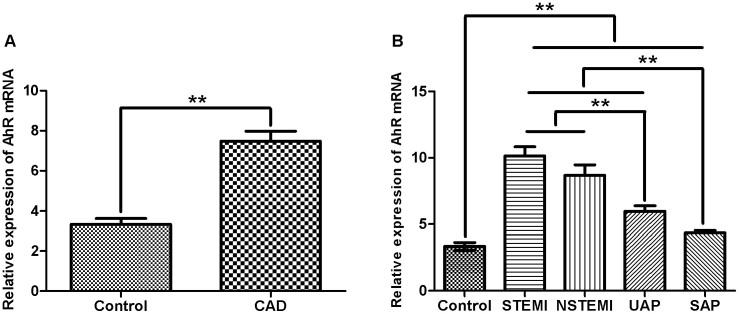
Peripheral blood AhR mRNA level were increased significantly by 2.197-folds in CAD patients compared with the controls (P < 0.001) (Figure 1A), as well as in each subtype of CAD compared with the control group, including ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), unstable angina pectoris (UAP) and stable angina pectoris (SAP) (P < 0.05) (Figure 1B). These results suggested that circulating AhR expression level was related to CAD pathogenesis.
Figure 1. Relative expression of AhR mRNA in patients.
(A): Comparison of AhR levels between CAD and control groups; (B): AhR levels from controls and patients with different CAD severities. β-actin expression was used as the internal control for quantitative analysis, and relative expression levels of target mRNA were calculated using the 2−ΔΔCt method. A total of 30 blood samples were tested in each experiment, which was carried out in triplicate. The histogram shows the mean ± standard error of the mean (SEM). One-way ANOVA was used to determine the significance of the differences. **P < 0.01 (ANOVA).
After a comparison between the four subgroups of CAD, the AhR level in acute coronary syndromes, STEMI, NSTEMI and UAP were all higher than that of SAP, while patients with STEMI and NSTEMI demonstrated more expression than those with UAP. However, there was no marked difference between STEMI and NSTEMI in terms of AhR expression.
AhR as a diagnostic biomarker for CAD
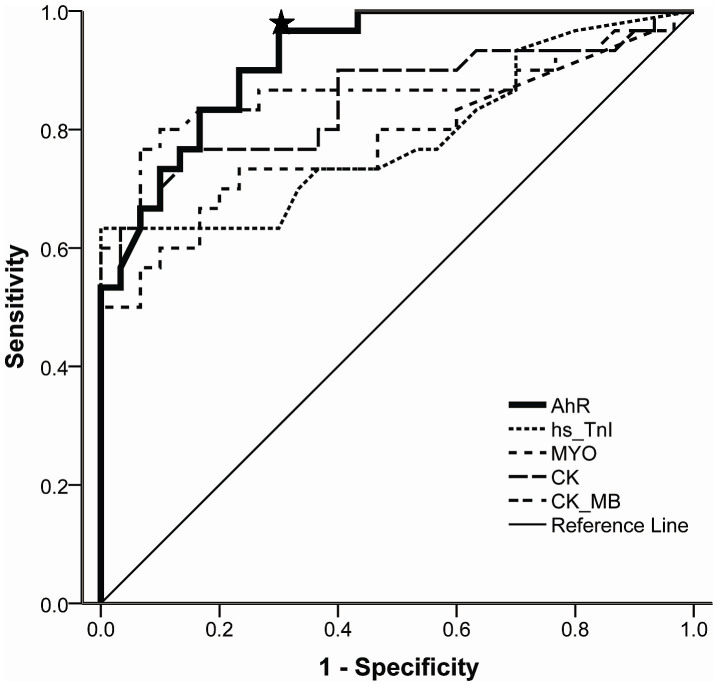
Receiver-operator characteristic (ROC) analysis was performed to evaluate the predictive power and diagnostic accuracy of circulating AhR for CAD. The area under ROC curve (AUC) was 0.921 (95% confidence interval (CI): 0.858–0.985, P < 0.0001), indicating that AhR has the potential to be a potent biomarker for CAD with a greater efficacy for the presence of CAD than hs-TnI (AUC 0.787; 95% CI 0.667–0.906), MYO (AUC 0.781; 95% CI 0.660–0.903), CK (AUC 0.849; 95% CI 0.747–0.952), or CK-MB (AUC 0.863; 95% CI 0.759–0.968) (Figure 2). A threshold expression of 4.025 AhR maximized true-positive and false-negative results (sensitivity 96.7%, specificity 70%).
Figure 2. Receiver operating characteristic (ROC) curves for AhR and other biomarkers in CAD patients.
The area under the ROC curve (AUC) of AhR was 0.921 (95% confidence interval (CI) 0.858–0.985). Asterisk indicates the threshold AhR mRNA level value of 4.025 that maximized true-positive and false-negative results (sensitivity 96.7%, specificity 70%). The AUC for high-sensitive troponin I (hs-TnI) was 0.787 (95% CI 0.667–0.906); that for myoglobin (MYO) was 0.781 (95% CI 0.660–0.903); that for creatine kinase (CK) was 0.849 (95% CI 0.747–0.952); and that for creatine kinase-isoenzyme (CK-MB) was 0.863 (95% CI 0.759–0.968).
The AhR polymorphism and CAD risk
Genotype and allele frequencies of AhR rs2066853 for CAD and control groups are shown in Table 2. GG is the wild-type genotype, followed by GA, which has a frequency about four-fold that of the AA genotype. G and A allelic frequencies were 66.61% and 33.39%, respectively, in CAD groups, and 62.84% and 37.15%, respectively, in the controls. The polymorphism passed the Hardy–Weinberg equilibrium (HWE) test for the distribution of genotypes in patients and controls (P > 0.05). Genotype frequencies of AhR were significantly different between CAD and control subjects, indicating that there was a remarkable association between the rs2066853 polymorphism and risk of CAD (P < 0.05).
Table 2. Genotype and allele frequencies of AhR polymorphisms between CAD and control groups.
| CAD group | Control group | ||||
|---|---|---|---|---|---|
| Genotype | n (%) | n (%) | AOR (95% CI) | P | P* |
| GG | 415 (44.2) | 334 (38.5) | 0.016 | 0.016 | |
| GA | 421 (44.8) | 423 (48.7) | |||
| AA | 103 (11.0) | 111 (12.8) | |||
| GG/GA versus AA | 836 (89.0) | 757 (87.2) | 1.19 (0.89–1.58) | 0.232 | 0.232 |
| GA/AA versus GG | 524 (55.8) | 534 (61.5) | 1.23 (1.05–1.53) | 0.014 | 0.014 |
| G allele | 1251 (66.6) | 1091 (37.2) | 1 | ||
| A allele | 627 (33.4) | 546 (37.2) | 0.85 (0.74–0.97) | 0.018 | 0.018 |
| χ2 | 1.645 | 0.06 | |||
| P | 0.2 | 0.807 |
Abbreviations: CAD, coronary arterial disease; AOR: adjusted odds ratio.
*False discovery rate-adjusted P value for multiple hypotheses test using BH method.
In the dominant genetic model (GG + GA versus AA), no significant difference was found between the CAD and control groups (P > 0.05), whereas the (GA + AA) combined genotype frequency of rs2066853 was significantly lower in CAD cases than controls (P < 0.05). When the GG genotype was used as a reference, a significantly decreased risk of CAD was associated with the GA (adjusted odds ratio (AOR) 0.801, 95% CI 0.658–0.976), AA (AOR 0.747 95% CI 0.551–1.013), and combined (GA + AA) genotypes (AOR 0.790 95% CI 0.655–0.953).
Association of the AhR polymorphism with the demographic characteristics
The stratification analyses of the AhR polymorphism and CAD risk are shown in Table 3. Samples were stratified according to different demographic characteristics such as age, gender, smoking, hypertension, diabetes, and hyperlipidemia. We found that the risk associated with the variant genotype of AhR rs2066853 increased significantly in males and patients less than 65 years of age (P < 0.05). Smoking also significantly promoted the CAD risk relative to the AhR rs2066853 polymorphism (P < 0.05). After the subjects were stratified based on hypertension, diabetes, and hyperlipidemia, an increased risk between cases and controls was observed in hyperlipemic, non-hypertensive, and non-diabetic individuals.
Table 3. Stratification analysis of AhR genotype and CAD risk.
| CAD group | Control group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype n(%) | Allele n(%) | Genotype n(%) | Allele n(%) | P value | |||||||||||
| Characteristics | GG | GA | AA | G | A | GG | GA | AA | G | A | Pg | Pg* | Pa | Pa* | AOR (95%CI) |
| Age | |||||||||||||||
| <65 years | 203(46.8) | 186(42.8) | 45(10.4) | 592(68.2) | 276(31.8) | 181(38.6) | 226(18.2) | 62(13.2) | 588(62.7) | 350(37.3) | 0.014 | 0.014 | 0.014 | 0.028 | 1.28(1.05–1.55) |
| ≥65 years | 212(42.0) | 235(46.5) | 58(11.5) | 659(65.2) | 351(34.8) | 153(38.3) | 197(49.4) | 49(12.3) | 503(63.0) | 295(37.0) | 0.319 | 0.319 | 0.329 | 0.658 | 1.10(0.91–1.34) |
| Gender | |||||||||||||||
| Male | 284(43.2) | 304(46.3) | 69(10.5) | 872(66.4) | 442(33.6) | 210(37.7) | 273(49.0) | 74(13.3) | 693(62.2) | 421(37.8) | 0.030 | 0.030 | 0.033 | 0.033 | 1.20(1.02–1.42) |
| Female | 131(46.4) | 117(41.5) | 34(12.1) | 379(67.2) | 185(32.8) | 124(39.9) | 150(48.2) | 37(11.9) | 398(64.0) | 224(36.0) | 0.246 | 0.492 | 0.245 | 0.245 | 1.15(0.91–1.47) |
| Smoking | |||||||||||||||
| Yes | 139(43.8) | 146(46.1) | 32(10.1) | 424(66.9) | 210(33.1) | 40(33.3) | 61(50.8) | 19(15.8) | 141(58.8) | 99(41.2) | 0.022 | 0.022 | 0.025 | 0.050 | 1.42(1.04–1.92) |
| No | 276(44.4) | 275(44.2) | 71(11.4) | 827(66.5) | 417(33.5) | 294(39.3) | 362(48.4) | 92(12.3) | 950(63.5) | 546(36.5) | 0.101 | 0.101 | 0.104 | 0.208 | 1.14(0.97–1.34) |
| Hypertension | |||||||||||||||
| Yes | 186(45.8) | 175(43.1) | 45(11.1) | 547(67.4) | 265(32.6) | 62(44.9) | 62(44.9) | 14(10.1) | 186(67.4) | 90(32.6) | 0.993 | 1.986 | 0.993 | 1.986 | 0.99(0.75–1.33) |
| No | 229(43.0) | 246(46.2) | 58(10.8) | 704(66.0) | 362(34.0) | 272(37.3) | 361(49.4) | 97(13.3) | 905(62.0) | 555(38.0) | 0.033 | 0.033 | 0.104 | 0.208 | 1.19(1.10–1.41) |
| Diabetes | |||||||||||||||
| Yes | 114(41.0) | 134(48.2) | 30(10.8) | 362(65.1) | 194(34.9) | 28(37.3) | 38(50.7) | 9(12.0) | 94(62.7) | 56(37.3) | 0.567 | 0.567 | 0.579 | 0.579 | 1.11(0.76–1.62) |
| No | 301(45.5) | 287(43.4) | 73(11.0) | 889(67.2) | 433(32.8) | 306(38.6) | 385(48.5) | 102(12.9) | 997(62.9) | 589(37.1) | 0.013 | 0.013 | 0.014 | 0.014 | 1.21(1.04–1.41) |
| Hyperlipidemia | |||||||||||||||
| Yes | 192(57.3) | 93(27.8) | 50(14.9) | 477(71.2) | 193(28.8) | 74(36.6) | 95(47.0) | 33(16.3) | 243(60.1) | 161(39.9) | 0.001 | 0.002 | <0.001 | <0.001 | 1.64(1.26–2.12) |
| No | 223(44.2) | 228(45.2) | 53(10.5) | 674(66.9) | 334(33.1) | 260(39.0) | 328(49.2) | 78(11.7) | 848(63.7) | 484(36.3) | 0.100 | 0.100 | 0.108 | 0.216 | 1.15(0.97–1.37) |
Abbreviations: CAD, coronary arterial disease; AOR: adjusted odds ratio; CI: confidence interval. Pg: P value of difference in genotypes between case and control groups. Pa: P value of difference in alleles between case and control groups.
*False discovery rate-adjusted P value for multiple hypotheses test using BH method.
Association of the AhR polymorphism with clinical indictors
To further determine the role of the AhR polymorphism in CAD occurrence, it was necessary to explore the relationship between the target genotype and CAD risk factors. Thus, we analyzed some common clinical indictors, including body mass index (BMI), blood glucose, CHOL, TG, HDL, and LDL, for their association with the AhR polymorphism. As shown in Table 4, CAD patient GG carriers of rs2066853 were associated with lower levels of HDL and higher LDL compared with controls. GA carriers had a significant correlation with higher LDL levels, but there was no significant difference between the AA genotype and all indicators. This finding was consistent with our earlier observation of a significant correlation between the AhR polymorphism and CAD risk among hyperlipidemic patients.
Table 4. Quantitative traits stratified by AhR genotypes.
| CAD group | Control group | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | GG | GA | AA | GG | GA | AA | PGG | PGG* | PGA | PGA* | PAA | PAA* |
| BMI | 23.2 ± 2.39 | 23.03 ± 2.38 | 23.25 ± 2.39 | 22.7 ± 2.4 | 22.95 ± 2.46 | 21.95 ± 4.39 | 0.034 | 0.051 | 0.7 | 0.7 | 0.023 | 0.069 |
| Glu (mmol/L) | 6.22 ± 4.22 | 6.5 ± 6.27 | 7.58 ± 12.06 | 5.85 ± 2.12 | 5.87 ± 1.87 | 5.73 ± 1.84 | 0.263 | 0.263 | 0.152 | 0.456 | 0.247 | 0.371 |
| CHOL (mg/dL) | 4.91 ± 1.26 | 4.91 ± 1.24 | 4.99 ± 1.56 | 4.78 ± 1.18 | 4.83 ± 1.23 | 4.88 ± 1.27 | 0.244 | 0.732 | 0.441 | 0.662 | 0.655 | 0.655 |
| TG (mmol/L) | 1.71 ± 1.23 | 1.55 ± 0.84 | 1.75 ± 1.19 | 1.52 ± 1.14 | 1.47 ± 1.03 | 1.47 ± 1.18 | 0.083 | 0.249 | 0.317 | 0.317 | 0.159 | 0.239 |
| HDL (mmol/L) | 1.28 ± 0.37 | 1.28 ± 0.37 | 1.31 ± 0.41 | 1.36 ± 0.42 | 1.32 ± 0.41 | 1.38 ± 0.37 | 0.03 | 0.09 | 0.113 | 0.17 | 0.336 | 0.336 |
| LDL (mmol/L) | 2.9 ± 1.16 | 2.88 ± 1.1 | 2.89 ± 1.11 | 2.61 ± 1.06 | 2.6 ± 1.17 | 2.58 ± 1.2 | 0.004 | 0.012 | 0.004 | 0.012 | 0.117 | 0.117 |
Abbreviations: CAD, coronary arterial disease; BMI, body mass index; Glu, blood sugar; CHOL, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein. Continuous data are expressed as the mean ± SEM. PGG: P value of GG genotype between case and control groups. PGA: P value of GA genotype between case and control groups. PAA: P value of AA genotype between case group and control group.
*False discovery rate-adjusted P value for multiple hypotheses test using BH method.
Association of the AhR polymorphism with CAD subtypes
The CAD cases in this study comprised the four major subgroups STEMI, NSTEMI, UAP, and SAP. We analyzed the definite effect of the AhR polymorphism on specific CAD subtypes, and detected a significant association between the AhR variant distribution and CAD when stratified according to the four subtypes (P < 0.001, PBH < 0.001), while the combined genotype (GA + AA) was also markedly associated with the CAD classification of the case cohorts (P < 0.001, PBH < 0.001). Together, these findings indicated that the rs2066853 polymorphism was significantly associated with CAD of different severities.
Discussion
The incidence of cardiac diseases has been increasing in recent years among the environmentally exposed population, as shown by previous epidemiological research carried out on an Italian population exposed to dioxin22. As a key sensor for environmental dioxin pollutants, considerable evidence has suggested a role for AhR in the pathogenesis of several cardiovascular events, although a direct association between AhR and CAD remains unclear10,23. In this hospital-based case-control study, we report for the first time on an association between AhR and CAD and its subtypes, indicating that circulating AhR could be used as a biomarker for CAD. The sensitivity and specificity of circulating AhR were notably superior to those of clinical biomarkers such as hs-TnI, MYO, CK, and CK-MB (Figure 2). These biomarkers reflect myocardial damage, while AhR is closely related to multiple pathogenic factors of CAD, although it remains unclear whether AhR is directly responsible for CAD development, and no studies have previously examined the role of AhR variants in CAD risk3,24,25.
In the present study, we investigated the association of AhR rs2066853 with CAD susceptibility in the Chinese population. The rs2066853 G > A missense mutation is located in exon 10 of AhR, and results in an arginine to lysine change in the transcriptional activation binding domain of the AhR protein26. Consequently, the genetic stability of AhR rs2066853 impacts strongly on the structure and function of the encoded protein, thus accounting for certain individual susceptibilities of disease16,17,27,28. Previous studies on rs2066853 have focused mostly on cancer, but their conclusions were fairly inconsistent and controversial. A haplotype analysis revealed significant differences in AhR haplotype distributions between CAD cases and controls, while the A allele of rs2066853 conferred an increased risk of lung cancer among heavy smokers in a Chinese population16. By contrast, another study showed that AhR polymorphisms, including rs2066853, were not involved in lung cancer in the Japanese population29. Similar conflicting results were also observed with respect to breast cancer, indicating that variants of AhR rs2066853 were considered a risk factor for breast cancer in Thai women but not in a multiethnic cohort study17,30. These controversies may be attributed to differences of genotype frequencies in sample sizes and ethnic populations. Our present research was based on a large sample (n = 1807), and revealed a significant correlation between rs2066853 and the risk of CAD in the Chinese population.
The pathogenic mechanism of CAD is very complex, and several clinical subtypes exist based on the degree of severity and clinicopathological characteristics. Optimal treatment relies on the discrimination of these different subtypes. In our study, the CAD subjects were divided into four subgroups, STEMI, NSTEMI, UAP, and SAP, and circulating AhR levels and the distribution of AhR rs2066853 variants were found to differ significantly between these subtypes. One of the pathogenetic differences of the four subtypes is atherosclerotic plaque stability31. STEMI, NSTEMI, and UAP are acute coronary syndromes that show unstable plaques, while plaques are stable in SAP32,33. A recent study showed that AhR contributed to plaque vulnerability and promoted atherosclerosis by inducing vascular inflammation11. Our present results revealed a close association between the AhR polymorphism and CAD susceptibility with different severities, which may be attributed to the effect of AhR on the vascular inflammatory response and plaque stability.
Some recent studies confirmed a higher incidence of hyperlipidemia and ischemic heart disease in workers exposed to dioxin and chlorinated organic compounds, suggesting that these AhR ligands cause hyperlipidemia34,35,36. Additionally, hyperlipidemia has been implicated in a significant reduction in LDL binding to its receptor on the hepatic plasma membrane when treated by TCDD37. Here, we demonstrated an association of AhR with hyperlipidemia and LDL, and showed that the AhR variant notably increased the CAD risk in hyperlipemia. As a crucial inflammatory mediator, AhR plays an important role in the TCDD-induced accumulation of cholesterol and lipids in macrophages, which was found to reflect the dose-dependent increase in chemokines such as interleukin 8, monocyte chemoattractant protein-1, and cyclooxygenase 238,39,40. Hence, AhR appears to mediate the development of dyslipidemia, indicating that its polymorphisms could be associated with CAD through the induction of hyperlipidemia.
AhR has a long evolutionary history and is ubiquitous in many human tissues, implying that this protein has an important role in growth and development26,41,42. It has also been shown to have a prominent biological function in cigarette smoke-mediated lung fibroblast and inflammation43. Moreover, a SNP study of 500 lung cancer patients and 517 cancer-free controls suggested that AhR polymorphisms and potential gene–smoking interactions may be involved in the pathogenesis of lung cancer16. In the current paper, we found that smoking markedly promoted the risk of CAD in relation to AhR rs2066853, which revealed a role for AhR in the CAD pathogenic mechanism and a new insight into the etiological relationship between smoking and cardiovascular diseases. Taken together, AhR may be one of the strongest candidate genes for susceptibility of tobacco-associated diseases.
In conclusion, AhR expression levels and functional AhR polymorphisms appear to affect the susceptibility and progression of CAD in Chinese populations, which increases our understanding of the mechanism by which dioxin pollution and cigarette smoke trigger cardiovascular diseases. These results provide a novel view into the etiology and epidemiology of CAD, and furthermore will contribute to the diagnosis and therapy of this severe disease. However, multicenter, parallel-group, and stratified randomization clinical trials will be required to confirm these findings, and more work is needed to elucidate the biological function and molecular mechanism of AhR in the CAD pathogeny.
Methods
Study population
Informed written consent was obtained from all CAD and control subjects and the study protocol was approved by the Institutional Ethics Committee of the Affiliated Hospital of Guangdong Medical College. All experimental methods applied in this study were carried out according to approved guidelines.
A total of 939 patients with CAD and 868 control subjects were recruited from the Affiliated Hospital of Guangdong Medical College (Zhanjiang, China) between May 2011 and August 2013. The clinical diagnosis of CAD was performed as follows: existing myocardial infarction, treated by percutaneous coronary intervention or coronary artery bypass graft, more than 50% stenosis in at least one of the main coronary arteries demonstrated angiographically, together with physiological examination including increased hs-TnI, MYO, CK and CK-MB. According to the degree of severity and clinicopathological characteristics, the CAD group was divided into four subgroups: STEMI, NSTEMI, UAP, and SAP, based on the American College of Cardiology/American Heart Association Task Force on Practice Guidelines20,21. All controls from the Health Examination Center attended routine medical examinations, and had no clinical manifestation or history of cardiovascular disorders, family history of coronary heart disease, or abnormal electrocardiography. Those with acute or chronic infections, systemic inflammatory diseases, autoimmune diseases, blood diseases, severe liver or renal function defects, malignant tumors, heart failure, arrhythmia, cardiomyopathies, and hematologic disorders were excluded from the study.
Physiological assay and blood collection
All study participants attended a standard medical examination, including measurements such as BMI, Glu, CHOL, TG, HDL, HDL.
Smoking was grouped as never smoking and smoking based on self-reports. Blood pressure (BP) was measured using a standard mercury sphygmomanometer, and hypertension was defined as systolic/diastolic BP level ≥140/90 mm Hg. Diabetes mellitus was assessed according to American Diabetes Association criteria (2 h postprandial glucose ≥11.1 mmol/L). Hyperlipidemia was diagnosed by CHOL ≥5.17 mmol/L. BMI was calculated as the individual's body mass divided by the square of their height.
Genomic DNA isolation and genotyping
Human genomic DNA was isolated from peripheral blood using the TIANamp Blood DNA Kit (Tiangen Biotech, Beijing, China), according to the manufacturer's instruction.
Direct sequencing was used for genotyping and SNP analysis. A total of 10 ng of genomic DNA from each subject was used as a template to PCR-amplify DNA fragments of 150–250 bp containing AhR rs2066853 using the following primers: forward, 5′-GATTGATTTTGAAGACCTCA-3′ and reverse, 5′-CTGAAGGTATGAAGGGAG-3′. After purification and precipitation by polyethylene glycol electrophoresis, the DNA fragment underwent direct sequencing on an ABI 3130XL DNA sequence detector with the BigDye® Terminator v3.1 Kit (Applied Biosystems, Foster City, CA, USA). The result was documented and analyzed by the GeneMapper 4.0 system (Applied Biosystems).
Blood mononuclear cells isolation and total RNA extraction
Human peripheral blood mononuclear cells from participants were isolated by the density gradient centrifugation method using Lymphoprep™ buffer (Axia-Shield PoCAS, Oslo, Norway), and were stored at −80°C.
Total RNA was extracted from blood mononuclear cells using the RNAprep Pure Blood Kit (Tiangen Biotech, Beijing, China). All contaminating DNA was eliminated using RNase-Free DNase I buffer and washed repeatedly to obtain pure RNA samples. This was quantified by spectrophotometry and its integrity checked by agarose gel electrophoresis prior to use for cDNA manipulation.
AhR mRNA expression analysis by real-time PCR
RNA was reverse transcribed into cDNA using random primers and the First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). The constitutively expressed β-actin gene was used as the internal control. AhR mRNA levels were quantified by real-time quantitative polymerase chain reaction (RT-qPCR) using SYBR® premix Ex Taq (Takara, Dalian, China). Primers were synthesized by Sangon Biotechnology (Shanghai, China) as follows: AhR forward, 5′-ATTGTGCCGAGTCCCATATC-3′, and reverse, 5′-AAGCAGGCGTGCATTAGACT-3′; β-actin forward, 5′-GGCACAGTCAAGGCTGAGAATG-3′, and reverse, 5′-ATGGTGGTGAAGACGCCAGTA-3′.
The 20 μl RT-qPCR mixture was composed of template cDNA, SYBR® Green PCR Master Mix (2×), DNase-free water, and forward and reverse primers. The RT-qPCR reaction followed a temperature-time profile: denaturation at 95°C for 5 min, 40 cycles of 95°C for 30 s, 60°C for 15 s and 72°C for 32 s, and dissociation curves analysis at 60–95°C. Amplification reactions were performed using the LightCycler 480® II real-time PCR System (Roche Diagnostics, Penzberg, Germany), and data were collected and analyzed using LightCycler 480® software SW 1.5 (Roche Diagnostics). Relative expression levels of target mRNA were calculated using the 2−ΔΔCt method.
Statistical analyses
All data were shown as means ± standard errors or percentage frequencies. Statistical analyses were performed using SPSS (ver. 18.0, IBM, New York, NY, USA) and GraphPad (ver. 6.0, La Jolla, CA, USA) software. Allele frequencies of AhR were calculated based on subject genotypes, and differences in allele and genotype distributions between CAD and control groups were analyzed by binary logistic regression or the chi-squared test. Multiple comparison corrections were performed with a one-way ANOVA using Bonferroni correction. Two-tailed P values, odds ratios, and 95% confidence intervals are presented for all association tests. The association between the circulating AhR level and CAD susceptibility or its risk factors was estimated using a multivariate logistic regression model. The HWE test for the AhR SNP was evaluated using the chi-squared test. Haplotype analyses were carried out using Haploview software (ver. 3.2.0, Broad Institute, Cambridge, MA, USA). A value of P < 0.05 was considered statistically significant.
The Benjamini-Hochberg multiple test correction was used to control the false discovery rate in logistic regression, providing an assessment of the expected proportion of false positives. ROC analyses were performed with AhR levels plotted against CAD. The AUC was calculated to evaluate the predictive and diagnosis power of circulating AhR for CAD occurrence.
Author Contributions
S.H., W.L. and C.C. conceived and designed the experiments. S.H., X.S., Y.H., C.C. and Y.X. performed the experiments. Y.H., X.S. and W.L. analyzed the data. S.H., W.L., C.C., G.L. and J.L. contributed reagents/materials/analysis tools. W.L. and X.S. contributed to the writing of the manuscript.
Acknowledgments
This paper was supported by the Natural Science Foundation of Guangdong Province (S2013040012115), Foundation for Distinguished Young Talents in Higher Education of Guangdong (2013LYM_0036) and the National Natural Science Foundation of China (no. 81270212 and no. 81300035).
References
- Vogel C. F., Goth S. R., Dong B., Pessah I. N. & Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 375, 331–335 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. et al. ITE and TCDD differentially regulate the vascular remodeling of rat placenta via the activation of AhR. PloS One 9, e86549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N. The role of endogenous aryl hydrocarbon receptor signaling in cardiovascular physiology. J Cardiovasc Dis Res 2, 91–95 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N. T., Hanieh H., Nakahama T. & Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol 25, 335–343 (2013). [DOI] [PubMed] [Google Scholar]
- Prigent L. et al. The aryl hydrocarbon receptor is functionally upregulated early in the course of human T-cell activation. Eur J Immunol 44, 1330–1340 (2014). [DOI] [PubMed] [Google Scholar]
- Kerley-Hamilton J. S. et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci 126, 391–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. K. et al. Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension 51, 803–809 (2008). [DOI] [PubMed] [Google Scholar]
- Lund A. K., Goens M. B., Kanagy N. L. & Walker M. K. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol Appl Pharmacol 193, 177–187 (2003). [DOI] [PubMed] [Google Scholar]
- Thackaberry E. A., Gabaldon D. M., Walker M. K. & Smith S. M. Aryl hydrocarbon receptor null mice develop cardiac hypertrophy and increased hypoxiainducible factor-1alpha in the absence of cardiac hypoxia. Cardiovasc Toxicol 2, 263–274 (2002). [DOI] [PubMed] [Google Scholar]
- Vasquez A. et al. A role for the aryl hydrocarbon receptor in cardiac physiology and function as demonstrated by AhR knockout mice. Cardiovasc Toxicol 3, 153–163 (2003). [DOI] [PubMed] [Google Scholar]
- Wu D. et al. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol 31, 1260–1267 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y. W. et al. Association between apolipoprotein E gene polymorphism and the risk of coronary artery disease in Chinese population: evidence from a meta-analysis of 40 studies. PloS One 8, e66924 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands C. J., Staskal D. F., Gollapudi B. & Budinsky R. The human AHR: identification of single nucleotide polymorphisms from six ethnic populations. Pharmacogenet Genomics 20, 283–290 (2010). [DOI] [PubMed] [Google Scholar]
- Beischlag T. V., Luis M. J., Hollingshead B. D. & Perdew G. H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr 18, 207–250 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frericks M., Burgoon L. D., Zacharewski T. R. & Esser C. Promoter analysis of TCDD-inducible genes in a thymic epithelial cell line indicates the potential for cell-specific transcription factor crosstalk in the AhR response. Toxicol Appl Pharmacol 232, 268–279 (2008). [DOI] [PubMed] [Google Scholar]
- Chen D. et al. Association of human aryl hydrocarbon receptor gene polymorphisms with risk of lung cancer among cigarette smokers in a Chinese population. Pharmacogenet Genomics 19, 25–34 (2009). [DOI] [PubMed] [Google Scholar]
- Sangrajrang S. et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int J Cancer 125, 837–843 (2009). [DOI] [PubMed] [Google Scholar]
- Wang X. W. et al. The association of functional polymorphisms in the aryl hydrocarbon receptor (AHR) gene with the risk of vitiligo in Han Chinese populations. Br J Dermatol 166, 1081–1087 (2012). [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A., Gallicchio L., Miller S. R., Zacur H. A. & Flaws J. A. Genetic polymorphisms in the aryl hydrocarbon receptor signaling pathway as potential risk factors of menopausal hot flashes. Am J Obstet Gynecol 207, 202.e9–202.e18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg P. G. et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33, 2569–2619 (2012). [DOI] [PubMed] [Google Scholar]
- Thygesen K. et al. Third universal definition of myocardial infarction. Eur Heart J 33, 2551–2567 (2012). [DOI] [PubMed] [Google Scholar]
- Humblet O., Birnbaum L., Rimm E., Mittleman M. A. & Hauser R. Dioxins and cardiovascular disease mortality. Environ Health Perspect 116, 1443–1448 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Zhang Z. & Luo X. Roles of xenobiotic receptors in vascular pathophysiology. Circ J 78, 1520–1530 (2014). [DOI] [PubMed] [Google Scholar]
- Gitsioudis G., Katus H. A. & Korosoglou G. Assessment of coronary artery disease using coronary computed tomography angiography and biochemical markers. World J Cardiol 6, 663–670 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zethelius B., Johnston N. & Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation 113, 1071–1078 (2006). [DOI] [PubMed] [Google Scholar]
- Harper P. A., Wong J. Y., Lam M. S. & Okey A. B. Polymorphisms in the human AH receptor. Chem Biol Interact 141, 161–187 (2002). [DOI] [PubMed] [Google Scholar]
- Wong J. M., Okey A. B. & Harper P. A. Human aryl hydrocarbon receptor polymorphisms that result in loss of CYP1A1 induction. Biochem Biophys Res Commun 288, 990–996 (2001). [DOI] [PubMed] [Google Scholar]
- Wong J. M. et al. Ethnic variability in the allelic distribution of human aryl hydrocarbon receptor codon 554 and assessment of variant receptor function in vitro. Pharmacogenetics 11, 85–94 (2001). [DOI] [PubMed] [Google Scholar]
- Kawajiri K. et al. Polymorphisms of human Ah receptor gene are not involved in lung cancer. Pharmacogenetics 5, 151–158 (1995). [DOI] [PubMed] [Google Scholar]
- Le Marchand L., Donlon T., Kolonel L. N., Henderson B. E. & Wilkens L. R. Estrogen metabolism related genes and breast cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 14, 1998–2003 (2005). [DOI] [PubMed] [Google Scholar]
- Zhao K. et al. Autophagy of monocytes attenuates the vulnerability of coronary atherosclerotic plaques. Coron Artery Dis 24, 651–656 (2013). [DOI] [PubMed] [Google Scholar]
- Gavorník P. Unstable atherosclerotic plaque and acute coronary syndromes. Bratisl Lek Listy 98, 351–359 (1997). [PubMed] [Google Scholar]
- Gutstein D. E. & Fuster V. Pathophysiology and clinical significance of atherosclerotic plaque rupture. Cardiovasc Res 41, 323–333 (1999). [DOI] [PubMed] [Google Scholar]
- Flesch-Janys D. et al. Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. Am J Epidemiol 142, 1165–1175 (1995). [DOI] [PubMed] [Google Scholar]
- Arsenescu V., Arsenescu R. I., King V., Swanson H. & Cassis L. A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect 116, 761–768 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelclova D. et al. Lipid metabolism and neuropsychological follow-up study of workers exposed to 2,3,7,8- tetrachlordibenzop-dioxin. Int Arch Occup Environ Health 75, S60–S66 (2002). [DOI] [PubMed] [Google Scholar]
- Bombick D. W., Matsumura F. & Madhukar B. V. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes reduction in the low density lipoprotein (LDL) receptor activities in the hepatic plasma membrane of the guinea pig and rat. Biochem Biophys Res Commun 118, 548–554 (1984). [DOI] [PubMed] [Google Scholar]
- Vogel C. F., Sciullo E. & Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc Toxicol 4, 363–373 (2004). [DOI] [PubMed] [Google Scholar]
- Vogel C. F. et al. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect 113, 1536–1541 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podechard N., Le Ferrec E., Rebillard A., Fardel O. & Lecureur V. NPC1 repression contributes to lipid accumulation in human macrophages exposed to environmental aryl hydrocarbons. Cardiovasc Res 82, 361–370 (2009). [DOI] [PubMed] [Google Scholar]
- Hahn M. E. The aryl hydrocarbon receptor: a comparative perspective. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 121, 23–53 (1998). [DOI] [PubMed] [Google Scholar]
- Karchner S. I., Franks D. G., Powell W. H. & Hahn M. E. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J Biol Chem 277, 6949–6959 (2002). [DOI] [PubMed] [Google Scholar]
- Martey C. A., Baglole C. J., Gasiewicz T. A., Sime P. J. & Phipps R. P. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 289, L391–L399 (2005). [DOI] [PubMed] [Google Scholar]