Abstract
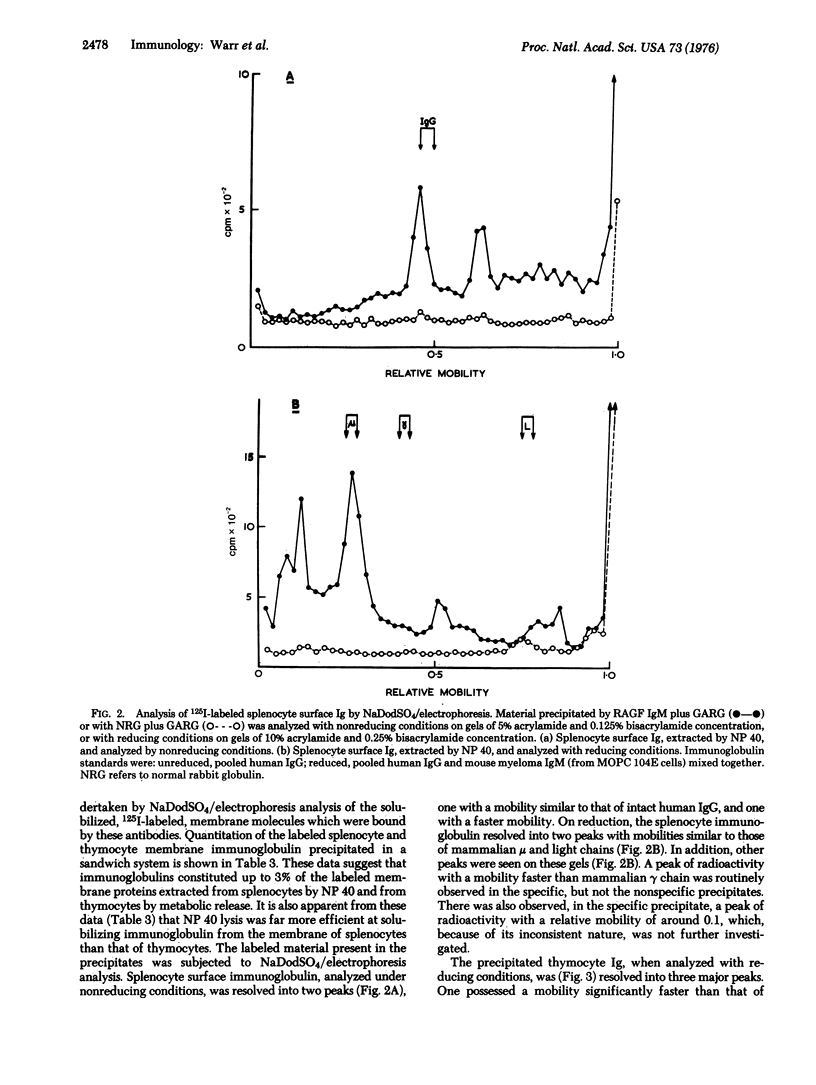
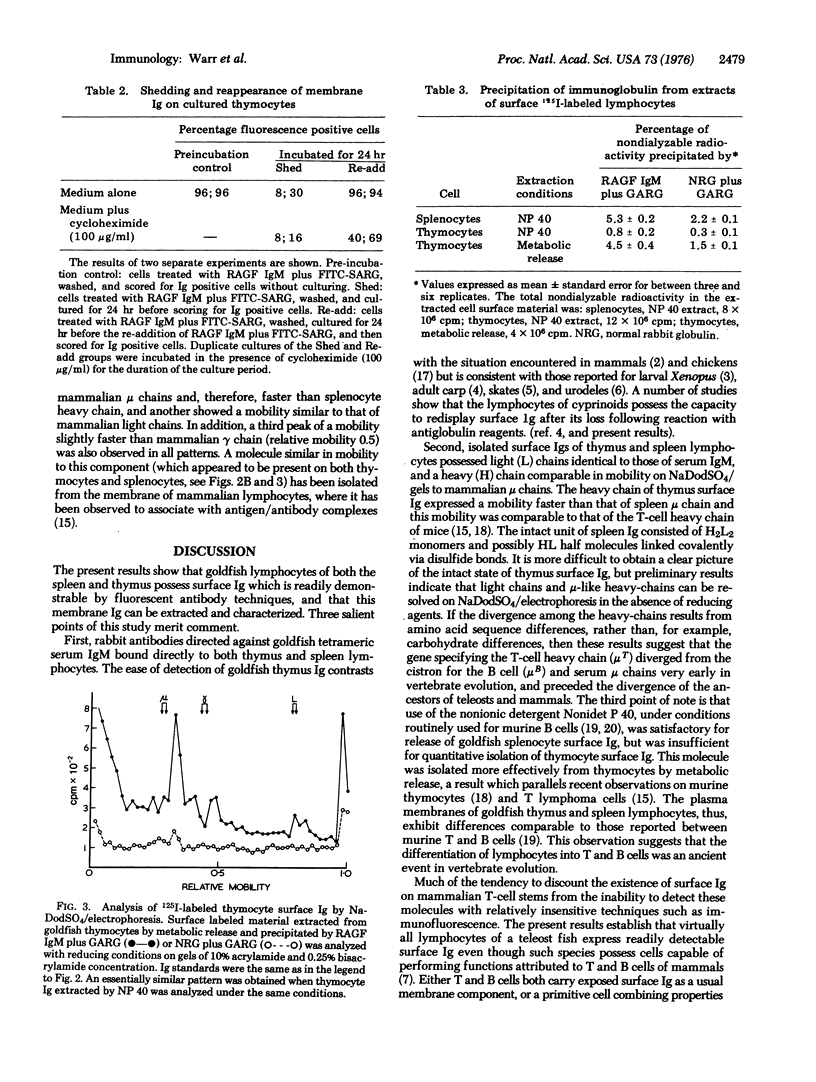
Membrane immunoglobulin (Ig) of splenocytes and thymocytes of the goldfish, Carassius auratus, was demonstrated by indirect fluorescent-antibody techniques. Observations on shedding and resynthesis indicated that the thymocyte Ig was endogenously produced. The lymphocyte surface proteins were radioiodinated using the lactoperoxidase-catalyzed reaction, and the labeled Ig molecules were isolated by specific precipitation and analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. The IgM-like membrane Igs of splenocytes and thymocytes were shown to differ in their ease of solubilization with nonionic detergent, and in the sodium dodecyl sulfate/electrophoretic mobility of their heavy chains. The significance of these observations for the evolution of T-cell recognition is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown B. A., Cooper E. L. Immunological dichotomy in the larval bullfrog spleen. Immunology. 1976 Feb;30(2):299–305. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Charlemagne J., Tournefier A. Cell surface immunoglobulins of thymus and spleen lymphocytes in urodele amphibian Pleurodeles waltlii (Salamandridae). Adv Exp Med Biol. 1975;64:251–255. doi: 10.1007/978-1-4684-3261-9_25. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Marchalonis J. J. Surface proteins of thymus-derived lymphocytes and bone-marrow-derived lymphocytes. Selective isolation of immunoglobulin and the theta-antigen by non-ionic detergents. Biochem J. 1974 Jun;140(3):345–354. doi: 10.1042/bj1400345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier L., Weiss N., Loor F. Direct evidence for immunoglobulins on the surface of thymus lymphocytes of amphibian larvae. Eur J Immunol. 1972 Aug;2(4):366–370. doi: 10.1002/eji.1830020414. [DOI] [PubMed] [Google Scholar]
- Ellis A. E., Parkhouse R. M. Surface immunoglobulins on the lymphocytes of the skate Raja naevus. Eur J Immunol. 1975 Oct;5(10):726–728. doi: 10.1002/eji.1830051014. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Richter R. F., Ambrosius H. Immunoglobulin determinants on the surface of lymphoid cells of carps. Eur J Immunol. 1975 Jan;5(1):76–78. doi: 10.1002/eji.1830050118. [DOI] [PubMed] [Google Scholar]
- Haustein D., Goding J. W. Surface immunoglobulin heavy chains of murine splenocytes and thymocytes are different. Biochem Biophys Res Commun. 1975 Jul 22;65(2):483–489. doi: 10.1016/s0006-291x(75)80173-2. [DOI] [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Harris A. W. Immunoglobulin of T lymphoma cells. Biosynthesis, surface representation, and partial characterization. Biochemistry. 1975 May 6;14(9):1826–1834. doi: 10.1021/bi00680a004. [DOI] [PubMed] [Google Scholar]
- Hudson L. Immunoglobulin-bearing lymphocytes of the chicken. I. Heavy chain immunoglobulin commitment and organ distribution. Eur J Immunol. 1975 Oct;5(10):694–698. doi: 10.1002/eji.1830051009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. Isolation and partial characterization of immunoglobulins of goldfish (Carassius auratus) and carp (Cyprinus carpio). Immunology. 1971 Feb;20(2):161–173. [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. Lymphocyte surface immunoglobulins. Science. 1975 Oct 3;190(4209):20–29. doi: 10.1126/science.1101378. [DOI] [PubMed] [Google Scholar]
- Shelton E., Smith M. The ultrastructure of carp (Cyprinus carpio) immunoglobulin: a tetrameric macroglobulin. J Mol Biol. 1970 Dec 28;54(3):615–617. doi: 10.1016/0022-2836(70)90133-6. [DOI] [PubMed] [Google Scholar]
- Virella G., Coelho I. M. Unexpected mobility of human lambda chains in sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Immunochemistry. 1974 Mar;11(3):157–160. doi: 10.1016/0019-2791(74)90213-4. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Bianco C., Nussenzweig V., Uhr J. W. Cell surface immunoglobulin. IV. Distribution among thymocytes, bone mrrow cells, and their derived populations. J Exp Med. 1972 Jul 1;136(1):81–93. doi: 10.1084/jem.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner N. L. Membrane immunoglobulins and antigen receptors on B and T lymphocytes. Adv Immunol. 1974;19(0):67–216. doi: 10.1016/s0065-2776(08)60252-7. [DOI] [PubMed] [Google Scholar]
- Yocum D., Cuchens M., Clem L. W. The hapten-carrier effect in teleost fish. J Immunol. 1975 Mar;114(3):925–927. [PubMed] [Google Scholar]