Abstract
The human non-selective cation channel TRPM2 represents a mediator of apoptosis triggered by oxidative stress. The principal agonist ADP-ribose binds to the cytosolic domain of TRPM2, which is homologous to the human ADP-ribose pyrophosphatase NUDT9. To further elucidate the structure-function relationship of this channel, we characterised a TRPM2 orthologue from the cnidarian Nematostella vectensis, after its expression in a human cell line. This far distant relative shows only 31% total sequence similarity to hTRPM2, while its C-terminal domain has a greater resemblance to the NUDT9 enzyme. Current through nvTRPM2 was induced by ADPR, with a more pronounced sensitivity and faster kinetics than in hTRPM2. In contrast to hTRPM2, there was no response to H2O2 and hardly any modulatory effect by intracellular Ca2+. The deletion of a stretch of 15 residues from the NUDT9 domain of nvTRPM2, which is absent in hTRPM2, did not change the response to ADPR but enabled activation of the channel by H2O2 and increased the effects of intracellular Ca2+. These findings shed new light on the evolution of TRPM2 and establish nvTRPM2 as a promising tool to decipher its complex gating mechanisms.
The transient receptor potential (TRP) subfamily of tetrameric cation channels contains various members with unusual properties, most notably TRPM2 with its integrated enzyme domain1. The C-terminal region of TRPM2 is homologous to the human (h) enzyme NUDT9 which represents an adenosine 5′-diphosphoribose (ADPR) pyrophosphatase of the NUDIX hydrolase family2. Channel gating most likely requires the binding but not the cleavage of the principal agonist ADPR (EC50 of approximately 100 μM)1 because the NUDT9 domain of TRPM2 contains mutations essential for channel function that reduce the ADPRase activity of the hNUDT9 enzyme by two orders of magnitude3,4,5. Cellular ADPR is produced by NAD+ metabolising enzymes on the plasma membrane such as CD38 which also hydrolyses cyclic ADPR to ADPR6. During apoptosis the release of ADPR into the cytosol is also driven by the poly-ADPR polymerase (PARP) and poly-ADPR glycohydrolase (PARG) pathways7,8. Numerous experimental data have assigned an important physiological role for TRPM2 in type I and type II diabetes, inflammation, lysosomal Ca2+ release as well as in cardiovascular and neurodegenerative diseases that are closely associated with apoptosis (for reviews see refs 9, 10). Currently, the most accepted hypothesis for the stimulation of TRPM2 by oxidative stress is that oxidants induce the accumulation of intracellular ADPR, which in turn activates TRPM25. As an experimental model for oxidative stress, extracellular H2O2 is frequently utilised and has been shown to trigger TRPM2 activation11,12. There is a positive Ca2+-driven feedback loop during the activation of TRPM2 because Ca2+ entry through the channel pore sensitises TRPM2 to the activation by ADPR1,13,14,15, all of which contributes to a fatal increase in the intracellular Ca2+ concentration during apoptosis.
Currently, there is little information regarding the structural requirements for the unique activation of TRPM2, mostly due to a lack of adequate experimental strategies to properly address the question. Orthologues of other TRP channels have been successfully exploited to elucidate the widely divergent gating mechanisms within the family (e.g., ref. 16). From phylogenetic analyses, there is evidence that a TRPM2-like channel containing a NUDT9 homology region (NUDT9H) represents the archetypal TRPM channel of non-metazoan origin17. This has been concluded because TRPM2-like channels are the only representatives of the TRPM- subfamily in the unicellular choanoflagellate Monosiga brevicollis as well as in the cnidarian Nematostella vectensis17. Currently, Nematostella vectensis represents a popular model organism for the evolution of the nervous system18 and the immune system19 in which the human TRPM2 channel plays an important physiological role9,10.
The aim of the present study was the functional analysis of the TRPM2-like channel of Nematostella vectensis (nvTRPM2) after heterologous expression in a human cell line. Through the generation of channel chimeras between nvTRPM2 and hTRPM2, we could gain insight into the functional importance of preserved and altered sections of both channels, particularly into the NUDT9H domain and its relation to channel activation by ADPR, Ca2+ and H2O2. We report that this approach enables the identification of sequences critical for the response to each stimulus. In addition, our results are the first to provide evidence for a different physiological role of an ADPR-gated cation channel from an organism that is considered as a model system to study early metazoan evolution20,21.
Results
Heterologous expression of the TRPM2 orthologue
For the functional characterisation in vitro of a TRPM2- orthologue far distant from hTRPM2, we selected the TRPM2-like channel of the sea anemone Nematostella vectensis (jgi.Nemve1.248535|estExt_fgenesh1_pg.C_6220005)17 the genome of which has been fully sequenced20 and which is a model organism for comparative studies and development e.g. of the nervous system18. We decided for this open reading frame because it yields the maximum sequence coverage and sequence identity, when compared with hTRPM2. Furthermore, the culture and manipulation of Nematostella vectensis is now established in many laboratorys worldwide, which also enables the implementation of future in- vivo studies. Commercially available gene synthesis was used to generate the open reading frame consisting of 4656 base pairs (bp) of the selected nvTRPM2 channel (Supplementary Fig. 1) and additionally offers the possibility of modifying the codon usage22. Thereby, we ensured the best possible adaption to the mammalian expression system (HEK-293 cells) for our in- vitro studies with the TRPM2 orthologue without changing the original sequence of amino acid residues (aa).
Sequence characteristics of the nvTRPM2 channel
The selected nvTRPM2 channel (1551 aa) displays a total sequence identity of 31% to the hTRPM2 channel (1503 aa). The similarity is greatest in the N-terminal region upstream of the putative transmembrane segments (36% identity) and in the NUDT9H domain (39% identity), whereas the regions containing the transmembrane segments (25% identity) and the connecting linker to the NUDT9H domain (27% identity) are less conserved (Supplementary Fig. 2). The NUDT9H domain of nvTRPM2 (aa 1271–1551) shows 49% sequence identity to the corresponding sequence of the hNUDT9 enzyme (aa 59–350) which is significantly higher than between the hNUDT9 enzyme and NUDT9H (aa 1236–1503) of hTRPM2 (34%; Supplementary Fig. 3). Compared to the hNUDT9-enzyme, in both nvTRPM2 and hTRPM2, the putative ADPR binding domain3 of the NUDT9H domain is well conserved, including the critical residue N1326 of hTRPM24. However, the active site of the hNUDT9 enzyme containing the NUDIX box signature GX5EX7REUXEEXGU23 is slightly different in NUDT9H of nvTRPM2 and markedly different in NUDT9H of hTRPM2 (Supplementary Fig. 3). A short amino acid motif within the proximal part of the predicted pore loop contributes significantly to the Ca2+ permeation of TRPM channels17. In the nonselective hTRPM2 channel, this motif consists of the amino acid triplet glutamine-isoleucine-proline (QIP), whereas in nvTRPM2 it is changed to glutamate-leucine-phenylalanine (ELF; Supplementary Fig. 2). Therefore, the nvTRPM2 channel contains the signature of a highly Ca2+-permeable channel17.
As a striking difference to the primary structure of hTRPM2, the nvTRPM2 channel exhibits a longer S1–S2 linker region with a number of glutamate and lysine residues. Notably, this region shows significant similarity to the corresponding region of the hTRPM3 channel (Supplementary Fig. 4), which strengthens the hypothesis that a TRPM2-like channel represents a common ancestor of the contemporary TRPM- subfamily17.
Sensitivity to ADP-ribose, intracellular and extracellular Ca2+ and current kinetics
The functional characterisation of nvTRPM2 and hTRPM2 transiently expressed in HEK-293 cells was performed with whole-cell patch-clamp analysis and calcium imaging experiments. At moderately increased concentrations of ADPR (25–50 μM) and in the absence of Ca2+ (≤10 nM) in the pipette solution, nvTRPM2-transfected cells developed large currents immediately after breaking into the cell that returned to baseline within a few seconds (Fig. 1a). The amplitude of these currents was approximately equivalent to the current flow through hTRPM2 induced by a 10-fold higher concentration of ADPR (Fig. 1b). As a control, we confirmed that there was no current through nvTRPM2 in the absence of ADPR in the pipette solution or when the Ca2+-concentration was buffered to ≤10 nM with EGTA on both sides of the cell membrane (Fig. 1c, d), which is also a characteristic feature of hTRPM213,14. Accordingly, currents from both hTRPM2 and nvTRPM2 were elicited in divalent-free extracellular solution only when the ADPR-containing pipette solution was supplemented with 1 μM Ca2+ (Fig. 2a).
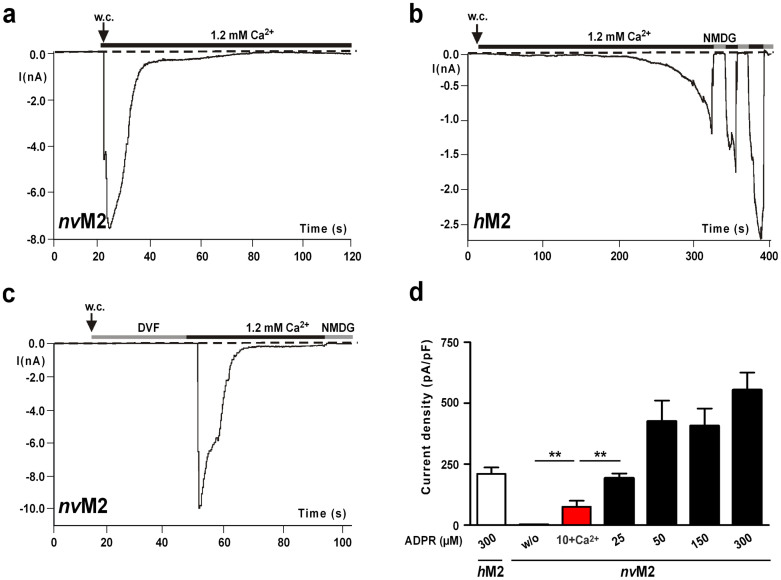
Figure 1. ADPR-dependent currents of nvTRPM2 and hTRPM2.
(a)–(c) Whole-cell patch-clamp measurements in HEK-293 cells. The pipette solution contained 50 μM ADPR (nvTRPM2) or 300 μM ADPR (hTRPM2). Intracellular Ca2+ concentration was adjusted to ≤10 nM. (a) Instantaneous current development of nvTRPM2 after reaching whole-cell configuration (w. c.) in the presence of 1.2 mM extracellular Ca2+. The currents spontaneously and rapidly decline to baseline. (b) Typical slow onset of currents of hTRPM2 recorded under the same experimental conditions as in panel a. Repeated current inhibition by substitution of external Na+ with the impermeable cation NMDG. (c) Same as in panel a, but the whole-cell configuration was established in extracellular divalent-free solution (DVF) in which Ca2+ was buffered with EGTA to ≤10 nM. Currents only developed after the extracellular DVF solution was substituted with standard bath solution containing 1.2 mM Ca2+. (D) Current densities in nvTRPM2 in dependence on the intracellular ADPR concentration. For experiments with 10 μM ADPR (highlighted in red), the intracellular Ca2+-concentration was adjusted to 1 μM, otherwise the intracellular Ca2+-concentration was below 10 nM. For comparison, corresponding data of hTRPM2 at 300 μM ADPR and unstimulated nvTRPM2 (w/o) are shown left. **Indicates a significant difference (P = 0.01) from Student's t-test. n = 8–16. Error bars are s.e.
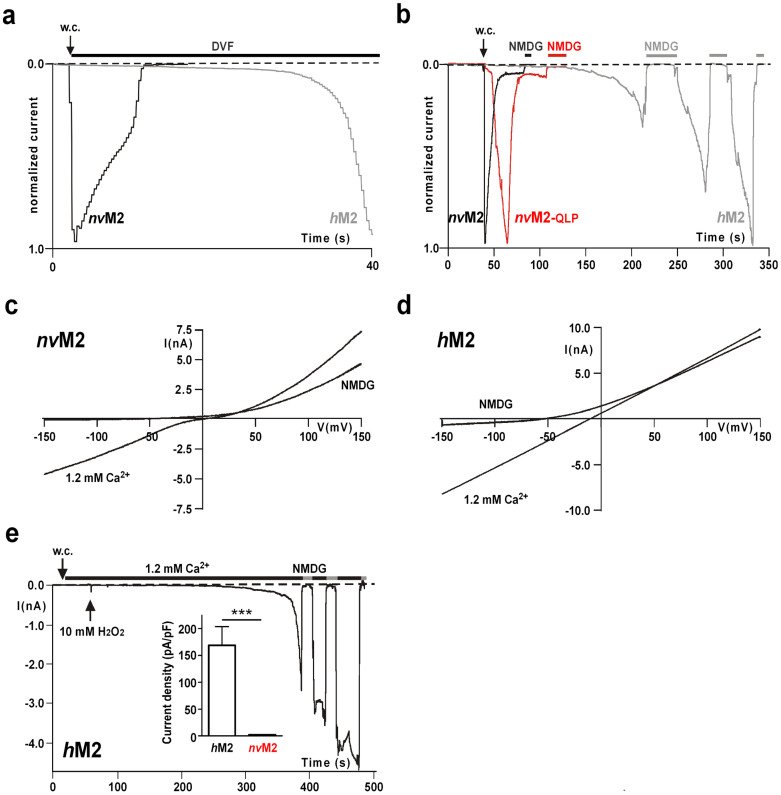
Figure 2. Current characteristics of nvTRPM2, nvTRPM2-(QLP) and hTRPM2 and sensitivity to H2O2.
(a) Normalized and superimposed current traces of wild-type nvTRPM2 and hTRPM2. Each current trace was obtained in a separate experiment by stimulation with ADPR (50 μM) and Ca2+ (1 μM) in the pipette solution. The extracellular solution was always divalent free. For each variant similar results were obtained from a least 4 independent experiments. (b) Normalized and superimposed current traces of wild-type nvTRPM2, nvTRPM2 variant (with pore signature ELF changed to QLP) and hTRPM2. Each current trace was obtained in a separate experiment by stimulation with ADPR (100 μM) and ≤10 nM Ca2+ in the pipette solution. Currents were initiated by exchanging the extracellular divalent-free solution with standard bath solution containing 1.2 mM Ca2+. Current flow was inhibited by superfusion of the cells with a solution containing NMDG as main cation. For each variant similar results were obtained from a least 5 independent experiments. (c, d) Representative current-voltage relations obtained from whole-cell patch clamp experiments of nvTRPM2 (c) and hTRPM2 (d). Recordings were performed either in standard bath solution (1.2 mM Ca2+) or in external solution containing NMDG as main cation. (e) Typical current development of hTRPM2 during superfusion with standard bath solution containing 10 mM H2O2 (time point of application indicated by an arrow). Intracellular concentration of Ca2+ was 1 μM. Currents were repeatedly inhibited by NMDG. Data on hTRPM2 and nvTRPM2 are summarized in the inset figure. ***Indicates a significant difference (P = 0.001) from Student's t-test. n = 9–14. Error bars are s.e.
To quantify the kinetics of inactivation of nvTRPM2, we measured the time over which 90% of the current decline occurred, which amounted to 14.1 ± 4 s (n = 7). This is in striking contrast to hTRPM2, which typically shows currents developing slowly and after a characteristic delay, depending on the intracellular concentrations of ADPR and Ca2+ (refs 1, 13, 14, 15, 24). Moreover, the run-down of hTRPM2 currents occurred over several minutes rather than seconds and was frequently incomplete within the time frame of the experiments (Fig. 1b). To assess the kinetics of the current development in nvTRPM2 during stimulation with ADPR and without Ca2+ in the pipette, experiments were performed in divalent-free extracellular solution (DVF), and currents were initiated by superfusion of the cells with standard bath solution containing 1.2 mM Ca2+ (Fig. 1c). Even under these experimental conditions, the activation of nvTRPM2 showed no delay, again in distinct contrast to hTRPM214.
When the pipette solution contained 10 μM ADPR and 1 μM Ca2+, the currents of nvTRPM2 were significantly smaller than those observed under conditions in which the patch pipette contained 25 μM ADPR and ≤10 nM Ca2+ (Fig. 1d). Typically, the ADPR-dependent current development of hTRPM2 is accelerated by intracellular Ca2+ (e.g. ref. 1). This is demonstrated with experiments shown in Figures 2a and 2b in which currents were either stimulated with 50 μM ADPR and 1 μM Ca2+ in the pipette solution (Fig. 2a; extracellular solution divalent free) or with intracelluar 100 μM ADPR and Ca2+ buffered to below 10 nM (Fig. 2b; extracellular solution with 1.2 mM Ca2+). In contrast, the current kinetics of nvTRPM2 were indistinguishable under both experimental conditions (Fig. 2a, b). These findings suggest that there is no graded modulation by intracellular Ca2+ on nvTRPM2 under the conditions studied. However, because the characteristic pore motif (ELF) of nvTRPM2 indicates a high permeability for Ca2+, the local Ca2+ concentration at its proposed activating sites15 might be considerably higher in nvTRPM2 than in hTRPM2. Therefore, we generated a nvTRPM2 variant with the hTRPM2-like pore signature (QLP). This manipulation should reduce the Ca2+-permeability of nvTRPM2 to the corresponding level of hTRPM2, as others have demonstrated using a similar strategy17. The stimulation of hTRPM2, nvTRPM2 and nvTRPM2-(QLP) with 100 μM ADPR and ≤10 nM Ca2+ in the patch pipette elicited currents with clearly different kinetics (Fig. 2b). In these experiments, the initially divalent-free bath was replaced by a solution containing 1.2 mM Ca2+. In wild-type nvTRPM2, this induced an almost instantaneous current activation and a rapid inactivation, whereas the current onset of hTRPM2 was strongly delayed. The activation kinetics of the nvTRPM2-(QLP) variant were slightly slower compared with wild-type nvTRPM2, but were still considerably faster than in hTRPM2. The current amplitudes of wild-type nvTRPM2 and nvTRPM2-(QLP) evoked with various concentrations of intracellular ADPR (50–300 μM) were not noticeably different.
Therefore, Ca2+-influx through the channel pore of nvTRPM2 may accelerate the activation kinetics of the channel. This effect may be explained by yet unidentified Ca2+ activating sites near the pore15. Both, determination of the relative Ca2+ permeability of the QLP variant and the identification of these sites in nvTRPM2 and hTRPM2 need further experimental investigation.
In our experiments performed with standard bath solution containing 140 mM NaCl, 1.2 mM CaCl2 and 1.2 mM MgCl2, the current-voltage -relationship of wild-type nvTRPM2 and nvTRPM2-(QLP) displayed a weak double rectification (shown for wild-type nvTRPM2 in Fig 2c), in contrast to the strictly linear I/V-curve of hTRPM2 (Fig. 2d). The currents reversed at approximately 0 mV and their inward components were inhibited by the replacement of extracellular Na+ with NMDG (Fig. 2c, d).
Insensitivity of nvTRPM2 to oxidative stress induced by the extracellular application of H2O2
A key feature of all TRPM2 channel orthologues studied previously is their activation in response to oxidative stress11,12 that is experimentally simulated by the extracellular application of H2O2 (Fig. 2d). Currently the most accepted hypothesis is that H2O2 activates the channel indirectly through an accumulation of intracellular ADPR5. This view is supported by inside-out patch-clamp experiments in which H2O2 apparently had no direct effects on TRPM225. However, a recent study reported that H2O2 sensitises hTRPM2 to activation by physiological body temperature through the oxidation of a methionine residue localised in the N-terminus of the channel26. This specific methionine residue is also conserved in nvTRPM2 (Supplementary Fig. 2). Nevertheless, we could not activate nvTRPM2 with H2O2 even at concentrations of 10–20 mM and in the presence of 1 μM [Ca2+]i (inset Fig. 2d). Similarly, the variant nvTRPM2-(QLP) did not respond to H2O2 in either patch-clamp experiments (n = 4) or in calcium imaging experiments (n = 10).
Swapping of the NUDT9 domains of nvTRPM2, hTRPM2 and the hNUDT9 enzyme
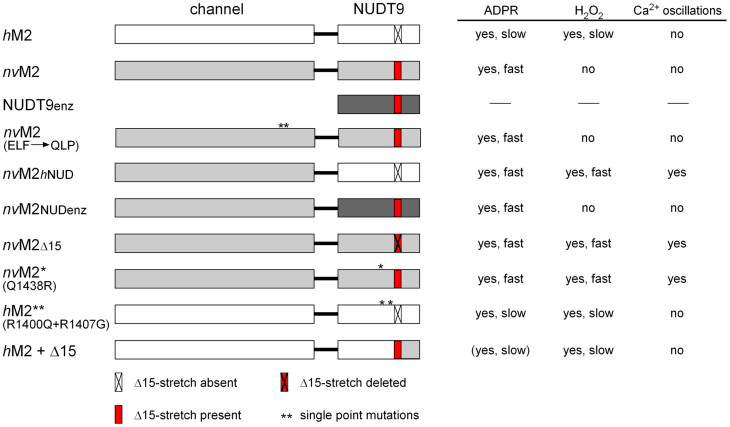
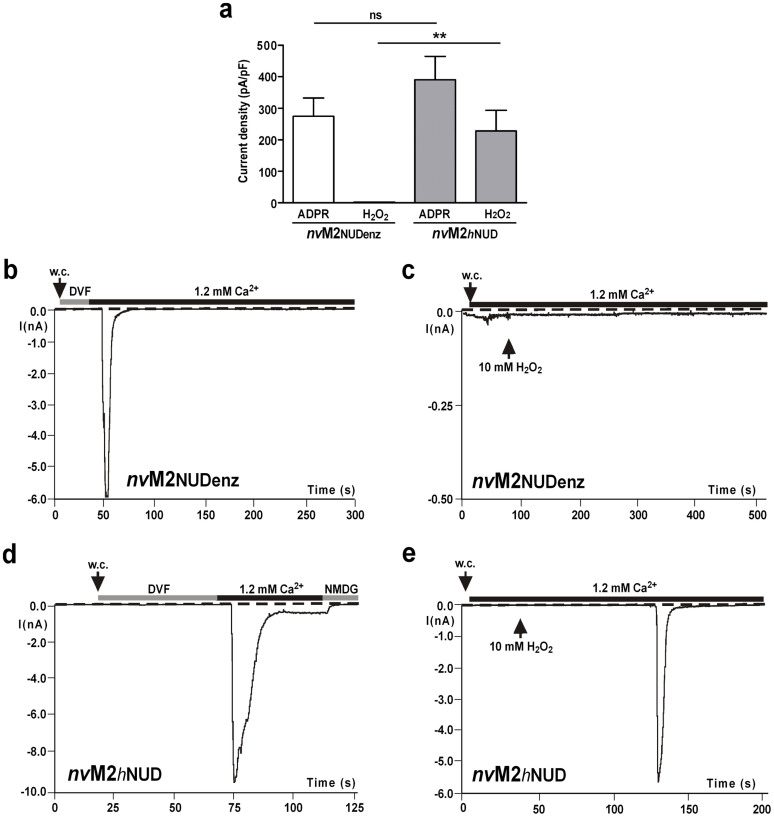
To further define the functional role of the NUDT9H domain, we generated chimeras of nvTRPM2 and hTRPM2 by exchanging the NUDT9H domains. The swapped NUDT9 sequences (hTRPM2: aa 1253–1503; nvTRPM2: aa 1289–1551) did not contain the functionally unimportant 60 N-terminal amino acid residues3. As a control, we also fused the corresponding part (aa 77–350) of the original hNUDT9 enzyme to each channel. An overview of the structure and the main functional properties of all TRPM2 variants analysed in this study is provided in Figure 3. As expected, the hTRPM2 channel variants that received the NUDT9 domain either from nvTRPM2 or from the hNUDT9 enzyme were inactive (Supplementary Fig. 5; no responses to 0.6 mM ADPR and 1 μM Ca2+ in the patch pipette or to extracellular stimulation with 10 mM H2O2), in line with previous results in which a partial restoration of the NUDIX consensus motif in hTRPM2 was incompatible with channel function4,5. By contrast, the nvTRPM2 chimera containing the central part of the hNUDT9 enzyme (nvTRPM2-NUDenz) was functionally indistinguishable from wild-type nvTRPM2. This includes the response to ADPR, the insensitivity to H2O2, the dependence on Ca2+ on at least one side of the cell membrane, as well as the gating kinetics (Fig. 4a–c).
Figure 3. Overview of all the TRPM2 variants analyzed in this study with schematic representation of their structure and main functional properties.
Figure 4. Sensitivity of nvTRPM2 chimeras with alternative NUDT9 domains to intracellular ADPR and extracellular H2O2.
(a) Average data of whole-cell patch clamp experiments where nvTRPM2-NUDenz (nvTRPM2 with NUDT9 domain of the hNUDT9 enzyme) or nvTRPM2-hNUD (nvTRPM2 with NUDT9 domain of hTRPM2) were stimulated either with 50 μM ADPR in the pipette solution (intracellular Ca2+-concentration ≤10 nM) or by extracellular application of 10 mM H2O2 (intracellular 1 μM Ca2+). **Indicates a significant difference (P = 0.01) from Student's t-test. n = 5–8. Error bars are s.e. (b)–(e) Representative recordings showing current development of nvTRPM2-NUDenz (b, c) and nvTRPM2-hNUD (d), (e) after stimulation with ADPR or H2O2 as indicated. During stimulation with ADPR, the experiments were started in divalent-free bath solution (DVF) and currents only developed after substitution of DVF with standard bath solution with 1.2 mM Ca2+.
Moreover, the nvTRPM2 chimera with the NUDT9H domain of hTRPM2 (nvTRPM2-hNUD) not only responded to ADPR in the same manner as wild-type nvTRPM2 (Fig. 4a, d) but had additionally gained sensitivity to H2O2 (Fig. 4a, e). HEK-293 cells expressing the nvTRPM2-hNUD chimera responded with large inward currents upon extracellular stimulation with 10 mM H2O2. Again, the activation kinetics were very fast, representing a typical feature of nvTRPM2. A quantitative analysis revealed that during the stimulation with 10 mM H2O2, the delay between the current onset and a current amplitude of 0.6 nA amounted to 3 ± 1.4 s (n = 7). The time over which 90% of the current had declined was 15.5 ± 12 s (n = 6). For comparison, the corresponding values for current development in hTRPM2 were 116 ± 54 s (n = 10; see Figs. 2d and 4e).
Identification of the NUDT9H sequences that confer sensitivity to H2O2
In the next step, we narrowed down the sequence requirements for the transfer of the H2O2 sensitivity from hTRPM2 to nvTRPM2. We initially concentrated on the NUDIX box and adjacent sequences. A detailed sequence comparison shows a deletion of a stretch of 14 or 15 amino acid residues slightly downstream of the NUDIX box in hTRPM2, compared to the nvTRPM2 channel and the hNUDT9 enzyme, respectively (Supplementary Fig. 3). Remarkably, this deletion is highly conserved in vertebrate TRPM2- ortholgues but absent in invertebrate TRPM2 channels. Furthermore, hTRPM2 contains several arginine residues (R1392, R1400, R1407) within the NUDIX domain where both nvTRPM2 and the hNUDT9 enzyme have neutral residues instead (Supplementary Fig. 3).
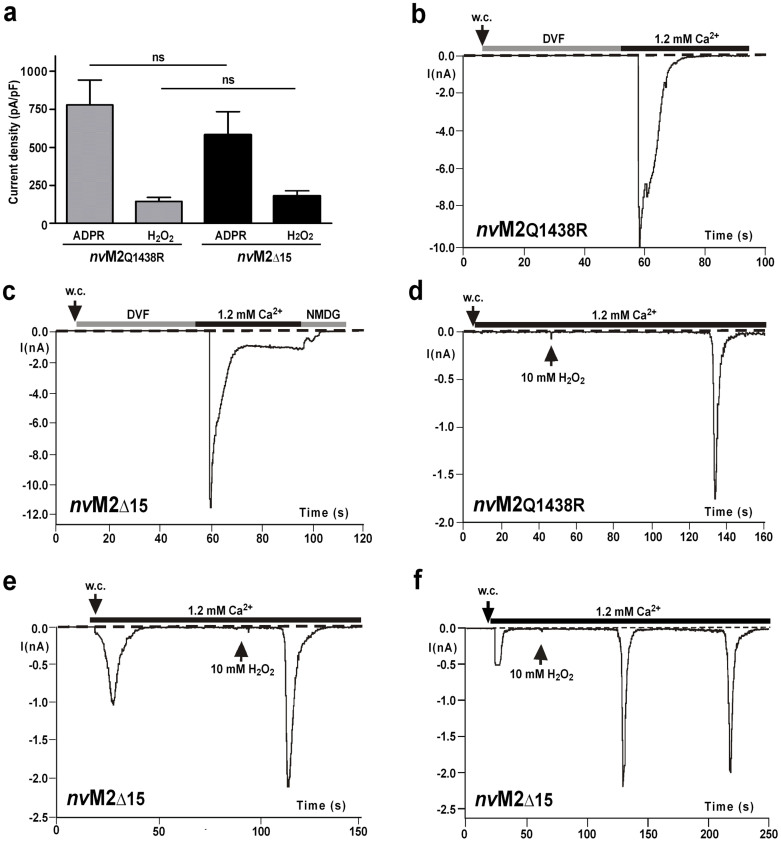
Therefore, we generated single point mutations in nvTRPM2 with arginine residues at the relevant positions as well as a nvTRPM2 variant with a corresponding deletion of the 15 amino acid residues downstream of the NUDIX box (Supplementary Fig. 3). The corresponding whole-cell patch-clamp data on nvTRPM2-(Q1438R) and nvTRPM2-(Δ15) are shown in Fig. 5. Similar to wild-type nvTRPM2, both variants displayed similar responses to ADPR as well as current inhibition when Ca2+ was removed from both sides of the cell membrane at the same time (Fig. 5a–c). Additionally, the two variants exhibited robust responses to H2O2 (Fig. 5a, d, e).
Figure 5. Sensitivity of nvTRPM2 variants with point mutation or deletion within the NUDT9 domain to intracellular ADPR and extracellular H2O2.
(a) Average data of nvTRPM2 variants either containing the point mutation Q1438R or a deletion of a stretch of 15 amino acid residues immediately downstream of the NUDIX box. Stimulation was performed with 50 μM intracellular ADPR or 10 mM extracellular H2O2 as described in Fig. 4. n = 4–9. (b)–(f) Representative recordings showing current development of nvTRPM2-Q1438R and nvTRPM2-(Δ15) after stimulation with ADPR (b), (c) or H2O2 (d), (e) as indicated. (f) Note the occurrence of spontaneous currents as well as the development of H2O2-induced repetitive currents in the variant nvTRPM2-(Δ15).
As an additional important finding, the nvTRPM2-(Δ15) channel exhibited transient spontaneous currents shortly after breaking into cell (Fig. 5e, f). These currents amounted to 65.2 ± 24 pA/pF, developed exclusively (n = 9 vs. n = 7 controls) in the presence of intracellular Ca2+ (1 μM in the pipette solution) and rapidly returned to baseline (Fig. 5e). The subsequent stimulation with H2O2 frequently evoked repetitive current developments that were interrupted by complete returns to baseline (Fig. 5f, n = 6 out of n = 9 experiments). As an additional control, neither spontaneous currents nor H2O2-induced currents were elicited when wild-type nvTRPM2 transfected HEK-293 cells were infused with a pipette solution containing 5 μM Ca2+ (n = 7).
H2O2-sensitive nvTRPM2 variants induce Ca2+-oscillations in HEK-293 cells
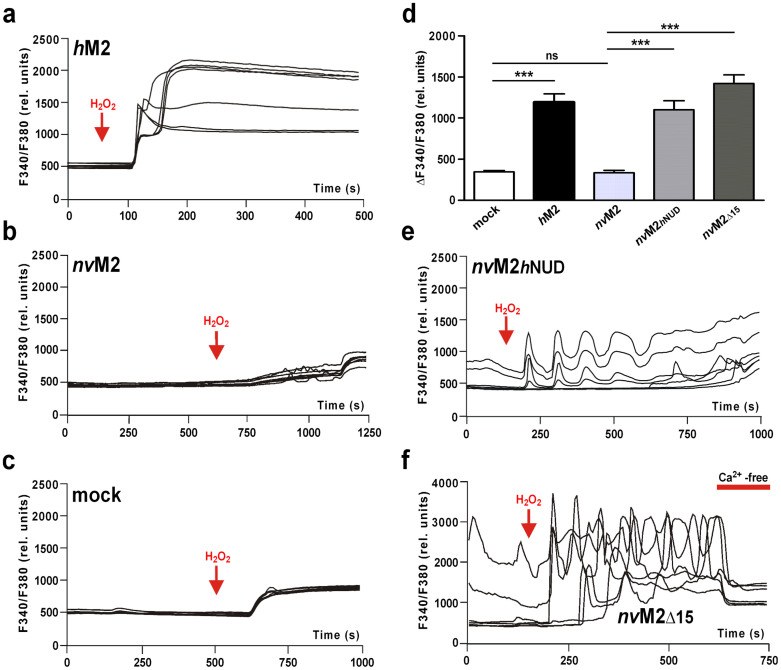
Because the electrophysiological experiments on nvTRPM2-(Δ15) revealed that a specific deletion of 15 amino acid residues modifies the nvTRPM2 channel from being solely sensitive to ADPR to being additionally sensitive to H2O2, we performed calcium imaging experiments with HEK-293 cells transfected either with nvTRPM2-(Δ15) or nvTRPM2-hNUD. The fact that these two nvTRPM2 variants can be stimulated by an extracellular stimulus, i.e., H2O2, is a prerequisite for this experimental approach. As positive control, we used wild-type hTRPM2, which showed a consistent and long-lasting increase in [Ca2+]i in response to H2O2 (Fig. 6a) as previously reported (e.g., ref. 27). By contrast, cells transfected with wild-type nvTRPM2 showed only a small increase in [Ca2+]i in response to H2O2, which was indistinguishable from those of mock-transfected cells (Fig. 6b–d). In nvTRPM2-hNUD, a considerable increase in [Ca2+]i was found after the application of 10 mM H2O2 (Fig. 6d, e). Remarkably, rather than a continuous rise in [Ca2+]i, a characteristic oscillation with an intermediate decline of [Ca2+]i relative to baseline was induced in the continuous presence of H2O2 (n = 8 out of 12 cells) (Fig. 6e). Only in nvTRPM2-(Δ15) did oscillations in [Ca2+]i sometimes develop spontaneously (n = 6 out of 19 cells) and were enhanced during stimulation with H2O2 (n = 16 out of 19 cells; Fig. 6f). The oscillations in [Ca2+]i demonstrate the presence of a positive feedback mechanism during the activation of specific nvTRPM2 variants that involves repetitive channel activation in the presence of intracellular Ca2+. This mechanism is best explained by a current enhancement by intracellular Ca2+, in line with the electrophysiological data.
Figure 6. Activity of H2O2-sensitive nvTRPM2 variants in measurements of intracellular Ca2+ (calcium imaging).
(a)–(c), (e), (f) Representative experiments on hTRPM2, nvTRPM2, empty vector control, nvTRPM2-hNUD and nvTRPM2-(Δ15) as indicated, showing the F340/F380 ratio over time. Extracellular H2O2 (10 mM) was added at time points marked with arrows. Maximal increases in F340/F380 are summarized in d. The inhibiting effect of extracellular Ca2+ removal is shown in panel f. ***Indicates a significant difference (P = 0.001) evaluated with a one-way ANOVA and the Bonferroni correction. n = 12–19. Error bars are s.e.
Mutation of critical NUDT9H sequences in hTRPM2
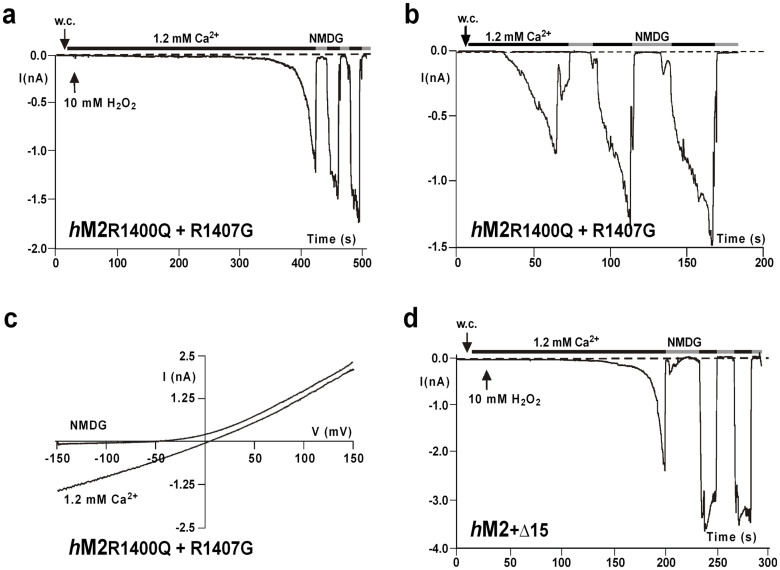
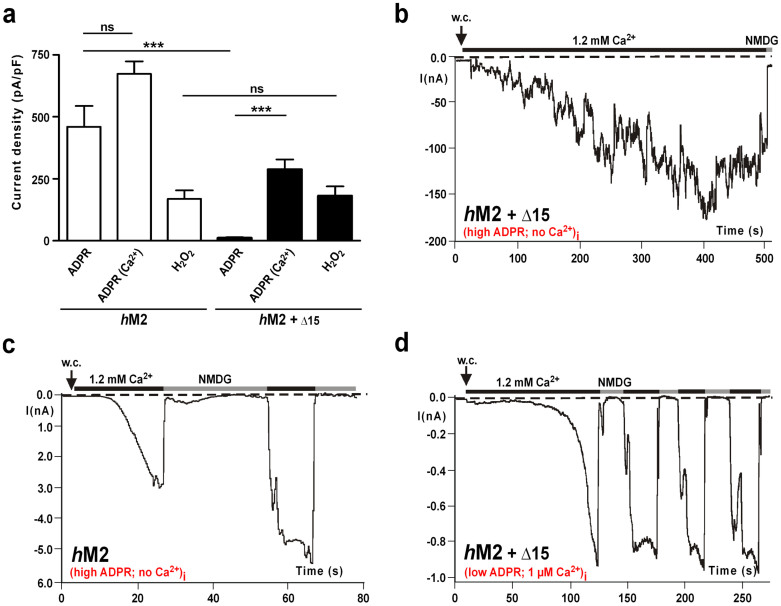
Because a few adjustments according to the sequence of hTRPM2 make the nvTRPM2 channel additionally sensitive to H2O2, it might be expected that responses to H2O2 are abolished or attenuated in the hTRPM2 orthologue with the opposite sequence manipulations. However, the sensitivity of the mutant hTRPM2-(R1400Q + R1407G) to H2O2 (Fig. 7a) and ADPR (Fig. 7b) as well as the I/V-curve (Fig. 7c) were unchanged, when compared with wild-type hTRPM2. As a further modification of hTRPM2, we replaced the entire C-terminal NUDT9H sequence downstream from the NUDIX box with the corresponding segment of nvTRPM2 (Supplementary Fig. 3). This segment also includes the sequence of 15 amino acid residues that is deleted in wild-type hTRPM2 (but not in the hNUDT9 enzyme) and is a potential determinant for the sensitivity to H2O2 and the additional effects of intracellular Ca2+. The variant hTRPM2-(+Δ15), in the presence of 1 μM [Ca2+]i, was as sensitive to H2O2 as wild-type hTRPM2 (Figs. 7d and 8a). However, the response to ADPR was significantly reduced when the intracellular co-agonist Ca2+ was buffered with EGTA to ≤10 nM (Fig. 8a, b). The presence of 1.2 mM ADPR in the pipette solution evoked only small currents in hTRPM2-(+Δ15), compared with wild-type hTRPM2, which displayed a strong and fast current development at similar concentrations (Fig. 8a, c). By contrast, the currents of hTRPM2-(+Δ15) in the presence of 1 μM [Ca2+]i were similar to those recorderd from hTRPM2, even at concentrations as low as 0.15 mM ADPR (Fig. 8a, d).
Figure 7. Sensitivity of hTRPM2 variants mutated wihin the NUDT9 domain, to intracellular ADPR and extracellular H2O2.
(a), (b) Representative whole-cell patch clamp recordings of hTRPM2 double mutant R1400Q + R1407G. Stimulation was performed (a) with extracellular 10 mM H2O2 or (b) with intracellular 0.3 mM ADPR. (c) Corresponding I/V-relation of currents recorded in panel b. (d) Representative whole-cell patch clamp recording of the variant hTRPM2-(+Δ15) that contains the C-terminal part of the NUDT9 domain of nvTRPM2 downstream of the NUDIX box. Stimulation was performed with extracellular 10 mM H2O2. Currents were repeatedly inhibited by NMDG.
Figure 8. Sensitivity of the hTRPM2 variant hTRPM2-(+Δ15) to intracellular ADPR in the presence or absence of intracellular free Ca2+.
(a) Average data of wild-type hTRPM2 and hTRPM2-(+Δ15) under stimulation with either intracellular ADPR (1.2 mM + ≤10 nM [Ca2+]i or 0.15 mM + 1 μM [Ca2+]i) or extracellular 10 mM H2O2. ***Indicates a significant difference (P = 0.001) from Student's t-test. n = 6–11. Error bars are s.e. (b)–(d), Representative whole-cell patch clamp recordings on hTRPM2-(+Δ15) and wild-type hTRPM2 as indicated, either stimulated with 1.2 mM intracellular ADPR in the presence of ≤10 nM Ca2+ in the patch-pipette (b), (c) or with 0.15 mM ADPR in the presence of 1 μM Ca2+ in the patch pipette (d).
Discussion
So far, the function of TRPM2 has been studied only with mammalian orthologues, in which its special role as a gateway for Ca2+ in the context of oxidative stress-mediated apoptosis was discovered7,11,26,28. Here, we have functionally expressed for the first time a TRPM2-like channel from Nematostella vectensis in a human cell line. The functional characterisation of this far distant relative of hTRPM2 facilitates new insights into the complex gating phenotype of the TRPM2 chanzyme. Currents through nvTRPM2 were induced by ADPR, with a greater sensitivity and faster kinetics than in the human orthologue, whereas the modulation provided by intracellular Ca2+ was less pronounced. In striking contrast to its effect on hTRPM2, the experimental trigger of oxidative stress, H2O2, did not have an effect on nvTRPM2. Using a chimeric approach, we identified some structural elements within the NUDT9 domain that conveyed sensitivity to H2O2 and modulated the effects of intracellular Ca2+ on nvTRPM2 as well as reduced the sensitivity to ADPR in hTRPM2. These findings further our understanding of the mechanisms involved in the complex gating process of hTRPM2 by various stimuli as well as the adaptions of this cation channel during evolution.
The current view of ADPR as a mediator of oxidative stress- induced apoptosis focuses on the role of mammalian TRPM2 channels as a self-reinforcing gateway of calcium influx26,28. It has been suggested that the stimulation of TRPM2 by oxidative stress is indirect, involving an intracellular accumulation of free ADPR5,25. Our present findings shed new light on the evolution of sensing oxidative stress because even low intracellular concentrations of ADPR evoke strong and immediate responses in nvTRPM2 that are characterised by a short duration, whereas H2O2 fails to stimulate the channel. By contrast, hTRPM2 has developed into a more polymodal channel with considerably slower kinetics and a distinct modulation by Ca2+. Presumably, the slower kinetics are a prerequisite for the ability of hTRPM2 to generate a self-reinforcing and sustained influx of Ca2+, which increases the susceptibility to cell death11.
Based on a comparative sequence analysis of hTRPM2 and nvTRPM2, we analysed the effects of the deletion of a stretch of 15 amino acid residues immediately downstream of the NUDIX box as well as the point mutation Q1438R within the NUDIX box. We found that both mutations convey H2O2 sensitivity to nvTRPM2. As a biological interpretation, by these changes hTRPM2 may have developed into a channel responsive to oxidative stress in a more versatile way than nvTRPM2 because it reacts to additional and possibly more direct signals than solely to ADPR. These data, especially on the nvTRPM2-(Δ15) variant, imply that the sensitivity to H2O2 is somehow crucially linked to intracellular Ca2+, but this hypothesis requires extensive future work. It is likely that further genetic alterations with the same effect exist because the reverse changes in hTRPM2, i.e., the chimera hTRPM2-(+Δ15) and the point mutations R1400Q + R1407G, did not abolish its responses to H2O2.
Compared with hTRPM2, nvTRPM2 exhibits peculiar characteristics especially in the time courses of activation and inactivation. The hTRPM2 channel shows on-reactions with a characteristic delay and a gradual development of current, followed by a very slow inactivation. By contrast, the activation of nvTRPM2 occurs within a few seconds, after which a clear and rapid inactivation ensues. Importantly, these species-specific kinetics are preserved after all of the manipulations on the NUDT9 domain and additionally applies to the newly acquired H2O2 responses of particular nvTRPM2 variants. Therefore, it could be hypothesised that the different kinetics do not represent differences in the reactions of the NUDT9 domain to the stimuli but rather reflect differences in the mechanisms of how a stimulated NUDT9 domain enables gating of the channel pore. This interactive process of channel gating in TRPM2 remains unclear. Future studies on nvTRPM2 may help to improve our understanding of this issue.
A characteristic feature of hTRPM2 is its strong dependency on intracellular Ca2+ in that there is a dramatic increase in the sensitivity to ADPR by one or two orders of magnitude when [Ca2+]i is increased from 0 to 1 μM13,14. It has been suggested that this makes hTRPM2 a mostly [Ca2+]i-regulated channel in the presence of endogenous ADPR levels24. The hTRPM2 channel possesses at least one potential calmodulin binding domain29 and the facilitatory effect of [Ca2+]i was postulated to be mediated by calmodulin14,29. However, in nvTRPM2 neither the presumed calmodulin binding domain of hTRPM2 nor other putative calmodulin binding sites are present. The finding that the channel activation of both hTRPM2 and nvTRPM2 depends on Ca2+, at least on one side of the cell membrane, strongly suggests that the presumed Ca2+ activating sites in the vicinity of the hTRPM2 pore15 are also present in nvTRPM2. In line with this idea, our patch-clamp studies on nvTRPM2 variants with presumably different permeabilities for Ca2+ reveal a significant but relatively small positive feedback of Ca2+ on channel activity. So far, these results are compatible with the lack of a calmodulin binding domain in nvTRPM2. However, the nvTRPM2-(Δ15) variant that contains the specific deletion of 15 amino acid residues characteristic of hTRPM2 displayed currents clearly dependent on [Ca2+]i. Moreover, in calcium imaging experiments with HEK-293 cells transfected with nvTRPM2-hNUD or nvTRPM2-(Δ15), oscillations in [Ca2+]i occurred either spontaneously or after stimulation with H2O2. In light of the non-physiological condition of a cnidarian channel chimera in a human expression system, we did not analyse these oscillations in more detail. In any case, because positive as well as negative feedback mechanisms are prerequisites for oscillations, our results provide evidence that the effects of intracellular Ca2+ on nvTRPM2-(Δ15), even without the binding of calmodulin, are sufficiently strong to create the observed oscillations in [Ca2+]i. The rapid current inactivation of nvTRPM2 and its variants is a likely mechanism for the required negative feedback, which is lacking in the human orthologue. In our experiments we did not find any oscillations in [Ca2+]i. for hTRPM2. However, Ca2+-oscillations have already been described for TRPM2 endogenously expressed in RIN-5F cells11, although these observations were obtained under strongly different experimental conditions.
The calcium-imaging data demonstrate that nvTRPM2 is inactive in intact cells with physiological concentrations of intracellular ADPR and Ca2+. If the stimulation with extracellular H2O2 increased the intracellular level of ADPR, a robust activation should be induced; this was not the case. Moreover, small non-specific rises in [Ca2+]i induced by extracellular H2O2 were not sufficient to stimulate nvTRPM2.
The functional properties of nvTRPM2-(Δ15) prompted us to create the human variant hTRPM2-(+Δ15) that displays a much weaker sensitivity to ADPR than wild-type hTRPM2. This is a remarkable feature because reported manipulations of the NUDT9 sequence in hTRPM2 have either completely abolished responses to ADPR or left them unaltered4,5. Interestingly, the hTRPM2-(+Δ15) variant is mutated neither in the putative ADPR binding region nor in the catalytic domain within the NUDIX box, but instead within the C-terminal part of NUDT9. The strongly attenuated response of hTRPM2-(+Δ15) to intracellular ADPR was particularly pronounced in the absence of intracellular Ca2+. The presence of Ca2+ (1 μM) in the pipette, however, largely restored the ADPR-sensitivity to the wild-type level. These findings with hTRPM2-(+Δ15) fit the hypothesis that the affected region is involved in the process that achieves gating of the pore after the binding of ADPR and support the conclusions derived from the different kinetics of nvTRPM2 and hTRPM2, but this awaits additional experimental studies.
Using several experimental approaches with nvTRPM2 variants, we have shown that channel activation by H2O2 can be separated from and is not mediated by ADPR. Most strikingly, responses to H2O2 were absent in wild-type nvTRPM2, although the sensitivity to ADPR is considerably stronger than in hTRPM2. Only by making distinct changes in the NUDT9 domain, is sensitivity to H2O2 produced, without affecting the ADPR sensitivity. Similarly, the variant hTRPM2-(+Δ15) is as sensitive to H2O2 as wild-type hTRPM2 but responds considerably weaker to ADPR. On the other hand, it is remarkable that the sensitivity to H2O2 and the modulatory effects of intracellular Ca2+ change in parallel in all variants studied, such that these two activation pathways cannot be discriminated thus far.
We conclude that hTRPM2 and its most distant orthologue examined so far, nvTRPM2, have been sufficiently conserved during evolution such that nvTRPM2 can be functionally expressed in human cells. Moreover, the nvTRPM2 channel has been shown to be functionally compatible with the NUDT9 domains of hTRPM2 and the hNUDT9 enzyme. Recent studies have revealed a striking similarity between Nematostella vectensis and vertebrates in terms of genomic organisation and gene content20,21. This could be an indication that many fundamental cellular processes of vertebrates and cnidaria were already present in the eumetazoan ancestor. Accordingly, hTRPM2 could have evolved from an archaic channel that originally reacted to ADPR in a highly sensitive but temporary manner to one that permits sustained responses. This modification could be a crucial step for the integration of TRPM2 into complex cellular processes. Moreover, by subtle changes within the NUDT9 domain that were partly identified in our experiments, TRPM2 gains sensitivity to H2O2 independent of ADPR. Thereby, mammalian TRPM2 channels may respond to an expanded spectrum of messengers and signals of oxidative stress and enable cation influx and enhanced calcium entry.
Methods
Molecular cloning
The amino acid sequence of the TRPM2-like channel of Nematostella vectensis was retrieved from the genomic database JGI (http://www.jgi.doe.gov/). The corresponding cDNA (nvTRPM2; jgi.Nemve1.248535|estExt_fgenesh1_pg.C_6220005) was synthesized by MWG-Biotech (Ebersberg, Germany) as two independent fragments. The 5′-terminal fragment of 2939 bp starts with a restriction site for Asc I followed by the Kozak sequence gccacc and the start codon and ends with a restriction site for Hind III. The 3′-terminal fragment of 1743 bp starts with the Hind III restriction site and ends with a stop codon followed by a Xba I restriction site. The codon usage was adapted during synthesis to ensure optimal expression in HEK-293 cells and the DNA sequence (Supplementary Fig. 1) was verified by double-strand DNA sequencing by MWG-Biotech. The 5′-terminal fragment was subcloned via Asc I + Hind III into the modified pIRES-hrGFP-2a vector (Stratagene, La Jolla, CA, USA) that contains a unique Asc I site instead of a single Nhe I site as well as a unique Xba I site instead of a single Xho I site. Subsequently, the 3′-terminal fragment was added via a Hind III + Xba I cloning step. The cDNA of hTRPM2 was subcloned via Eco RI + Xba I into the pIRES-hrGFP-2a vector. The cDNA from the human NUDT9 enzyme (Accession No: NM_024047.3) was purchased from AMS Biotechnology, (Abingdon, UK). Site-directed mutagenesis was performed using the QuikChange mutagenesis system (Stratagene, La Jolla, CA, USA). Defined oligonucleotides were obtained from MWG-Biotech. Each point mutation and chimeric channel construct was verified by DNA sequencing (MWG-Biotech). All procedures were performed in accordance to the respective manufacturer's instructions, unless indicated otherwise. Sequence alignments were performed according to the UniProtKB alignment tool at www.uniprot.org.
Cell culture and transfection
HEK-293 cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and cultured in DMEM media (Biochrome, Berlin, Germany) supplemented with 4 mM L-glutamine and 10% (v/v) foetal calf serum (Biochrome) and 2 mM sodium pyruvate. Transient transfections of HEK-293 cells with the cDNAs of hTRPM2, nvTRPM2 or chimeric variants were performed using the FuGene 6 transfection reagent (Roche, Mannheim, Germany) according to the manufacturer's instructions. As controls, cells were transfected with the pIRES-hrGFP-2a vector alone. The transfected cells were maintained for 24 h in an incubator at 37°C and 5% CO2. Subsequently, the cells were seeded on poly-lysine-coated glass coverslips at a suitable dilution and further incubated for 3–4 h. Then, patch-clamp and calcium imaging experiments were carried out in cells visibly positive for EGFP. At least three independent transfections were used for each experimental group.
Electrophysiology
Whole-cell recordings were performed using an EPC 9 amplifier equipped with a personal computer with Pulse 8.5 and X Chart software (HEKA, Lamprecht, Germany). The standard bath solution contained (in mM) 140 NaCl, 1.2 MgCl2, 1.2 CaCl2, 5 KCl, 10 HEPES, pH 7.4 (NaOH). For Na+ free solutions, Na+ was replaced by 150 mM N-methyl-D-glucamine (NMDG) and the titration was performed with HCl. The divalent free bath solution (DVF) contained (in mM) 150 NaCl, 10 EGTA, 10 HEPES, pH 7.4 (NaOH). The pipette solution contained (in mM) 145 CsCl, 8 NaCl, 2 MgCl2, 10 HEPES, pH 7.2 (CsOH) and the Ca2+ concentration was adjusted to either ≤10 nM (10 mM Cs-EGTA, no Ca2+ addition), to 1 μM (0.886 mM Ca2+, 1 mM Cs-EGTA) or to 5 μM (0.970 mM Ca2+, 1 mM Cs-EGTA). The Ca2+ concentration of the solutions was calculated using the MAXC-program: (http://www.stanford.edu/~cpatton/maxc.html). For the stimulation of TRPM2, Adenosine diphosphate ribose (ADPR; 100 mM stock solution in distilled water) was added to the intracellular solution yielding a final concentration of 0.001-1.2 mM. Alternatively, TRPM2 currents were evoked by superfusion of the cells with standard bath solution containing 10 mM H2O2 (diluted from a 30% stock solution). Unless otherwise stated, the experiments were performed at room temperature (21°C) and the current-voltage relations were obtained during voltage ramps from −150 to +150 mV and back to −150 mV applied over 200 ms. The holding potential was −60 mV. For the analysis the maximal current amplitudes (pA) in a cell were divided by the cell capacitance (pF), a measure of the cell surface. The result is the current density (pA/pF).
Calcium imaging experiments
For fluorescence imaging of [Ca2+]i HEK-293 cells on poly-lysine-coated glass coverslips were loaded in standard bath solution containing membrane-permeable Fura-2 acetoxymethyl ester (1.5 ng/μl; Invitrogen) and pluronic acid (0,025%) for 20 min at 37°C. Fluorescence was alternately excited at 340 and 380 nm using the Polychrome IV monochromator (TILL Photonics). The emitted fluorescence was measured at 510 nm using a Sensicam (IMAGO). Fluorescence was corrected for background at each wavelength. Measurements were obtained at room temperature (21°C). The standard bath solution and stimulation with H2O2 were identical to those described for the patch-clamp experiments.
Data analysis and statistics
Data are expressed as the mean ± s.e.m.. Unless statet otherwise, the comparison of two groups was performed using an unpaired Student's t-test. Calcium imaging experiments were statistically evaluated using a one-way ANOVA and the Bonferroni correction was applied when multiple comparisons were performed with the same control data. Differences were considered significant at **P < 0.01 and ***P < 0.001.
Author Contributions
F.K. developed the concept, designed the study, performed all whole-cell patch clamp experiments, analysed the data and wrote the paper. C.K. conducted all calcium imaging experiments and analysed the data. A.L. was involved in data interpretation and preparing of the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Daniel C. Hoffmann and Marina Wolf for their help during the course of this study. The study was supported by the Deutsche Forschungsgemeinschaft (DFG KU 2271/4-1).
References
- Perraud A. L. et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 31, 595–599 (2001). [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Carloto A., Costas M. J. & Cameselle J. C. Human placenta hydrolases active on free ADP-ribose: an ADP-sugar pyrophosphatase and a specific ADP-ribose pyrophosphatase. Biochim Biophys Acta. 1526, 86–94 (2001). [DOI] [PubMed] [Google Scholar]
- Shen B. W., Perraud A. L., Scharenberg A. & Stoddard B. L. The crystal structure and mutational analysis of human NUDT9. J Mol Biol. 332, 385–398 (2003). [DOI] [PubMed] [Google Scholar]
- Kühn F. J. & Lückhoff A. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J Biol Chem. 279, 46431–46437 (2004). [DOI] [PubMed] [Google Scholar]
- Perraud A. L. et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 280, 6138–6148 (2005). [DOI] [PubMed] [Google Scholar]
- Howard M. et al. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 262, 1056–1059 (1993). [DOI] [PubMed] [Google Scholar]
- Fonfria E. et al. TRPM2 channel opening in response to oxidative stress is dependent on activation of poly(ADP-ribose) polymerase. Br J Pharmacol. 143, 186–192 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow B., Song Y. & Scharenberg A. M. The Poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem. 283, 24571–24583 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faouzi M. & Penner R. TRPM2. Handb Exp Pharmacol. 222, 403–426 (2014). [DOI] [PubMed] [Google Scholar]
- Ru X. & Yao X. TRPM2: a multifunctional ion channel for oxidative stress sensing. Sheng Li Xue Bao. 66, 7–15 (2014). [PubMed] [Google Scholar]
- Hara Y. et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 9, 163–173 (2002). [DOI] [PubMed] [Google Scholar]
- Wehage E. et al. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem. 277, 23150–23156 (2002). [DOI] [PubMed] [Google Scholar]
- McHugh D., Flemming R., Xu S. Z., Perraud A. L. & Beech D. J. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 278, 11002–11006 (2003). [DOI] [PubMed] [Google Scholar]
- Starkus J., Beck A., Fleig A. & Penner R. Regulation of TRPM2 by extra- and intracellular calcium. J Gen Physiol. 130, 427–440 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csanády L. & Törocsik B. Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate. J Gen Physiol. 133, 189–203 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva E. O. et al. Molecular basis of infrared detection by snakes. Nature 464, 1006–1011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos Y., Schnitzler M., Wäring J., Gudermann T. & Chubanov V. Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J. 22, 1540–1551 (2007). [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Renfer E., Technau U. & Rentzsch F. Nervous systems of the sea anemone Nematostella vectensis are generated by ectoderm and endoderm and shaped by distinct mechanisms. Development 139, 347–357 (2012). [DOI] [PubMed] [Google Scholar]
- Miller D. J. et al. The innate immune repertoire in cnidaria--ancestral complexity and stochastic gene loss. Genome Biol. 8, R59 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N. H. et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (2007). [DOI] [PubMed] [Google Scholar]
- Darling J. A. et al. Rising starlet: the starlet sea anemone, Nematostella vectensis. Bioessays 27, 211–221 (2005). [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 2, 13–34 (1985). [DOI] [PubMed] [Google Scholar]
- Bessman M. J., Frick D. N. & O'Handley S. F. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J Biol Chem. 271, 25059–25062 (1996). [DOI] [PubMed] [Google Scholar]
- Heiner I. et al. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J. 398, 225–32 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B. & Csanády L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. J Biol Chem. 285, 30091–30102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashio M. et al. Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc Natl Acad Sci U S A 109, 6745–6750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecquet C. M., Ahmmed G. U., Vogel S. M. & Malik A. B. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 102, 347–355 (2007). [DOI] [PubMed] [Google Scholar]
- Park L. et al. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat Commun. 5, 5318. 10.1038/ncomms6318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q. et al. Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem. 281, 9076–9085 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information